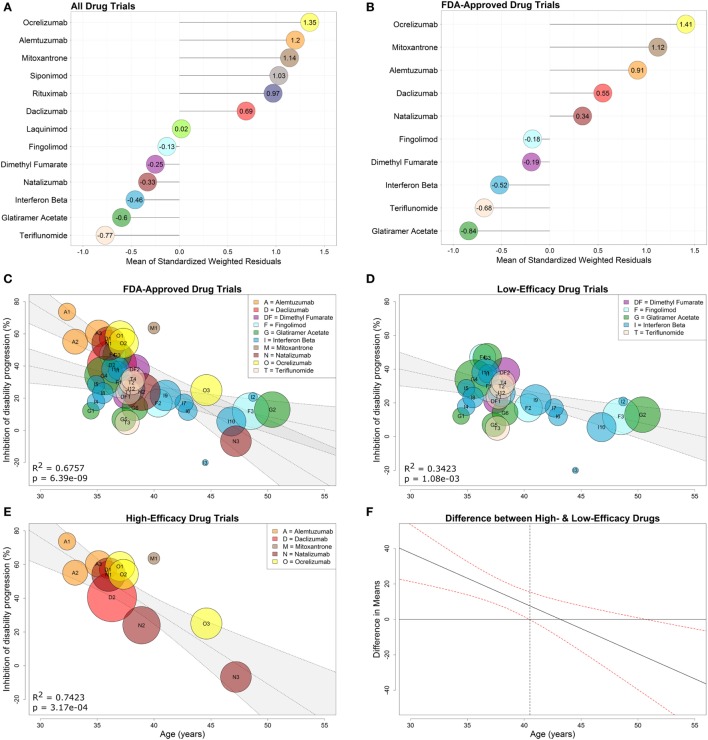
Figure 4.
Low- and high-efficacy categories derived from drug-specific weighted residuals and development of optimized model with interaction between age and efficacy. Comparative efficacy ranks for standardized, drug-specific weighted residual means computed from the linear regression fit to all drugs (A) or fit to clinical trials of FDA-approved drugs studied in FDA-approved indications (B). The means of the drug-specific residuals are provided directly in the lollipop plots. FDA-approved immunomodulatory disease-modifying therapies from (B) were then separated into high-efficacy drugs (i.e., drugs with positive means) and low-efficacy drugs (i.e., drugs with negative means). A regression model that includes all FDA-approved drugs with an interaction between age and efficacy (0 for low-efficacy, 1 for high-efficacy) is depicted in (C). Simple weighted linear regressions were fit to clinical trials of low-efficacy (D) and high-efficacy (E) drugs using only trials that studied FDA-approved drugs. Corresponding coefficients of determination (R2) and p-values are included in the individual plots, while the inset legends provide color and alphabet code for individual drugs. (F) The 95% confidence interval denotes the statistically significant difference in means between low- and high-drug efficacy as a function of age. The gray dashed vertical line indicates that there is no significant difference between low- and high-efficacy drugs past age 40.5 years.