Fig. 1.
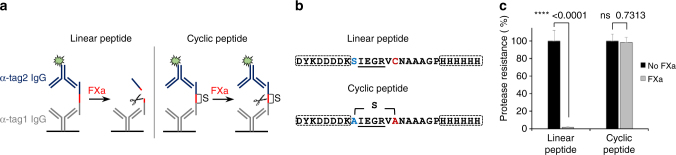
An ELISA-based reporter assay monitors the cyclization status of artificial lanthipeptides. a Peptides containing an FXa recognition site (red bar) flanked by affinity tags and residues involved in thioether bridge formation are captured via anti-tag1 antibodies, treated with FXa, and detected via anti-tag2 antibodies. Tag2 is proteolytically removed (left panel) in linear peptides, but remains connected via a covalent thioether (indicated by “S”, right panel) in cyclic peptides, resulting in low and high signals, respectively. b Sequences of synthetic linear or thioether-bridged peptides with FLAG- and His6-epitopes (boxed) flanking the FXa site (underlined) used for assay validation. c Synthetic peptides were captured via the His6-tag, incubated with or without FXa, and detected using anti-FLAG IgG. The protease resistance relative to untreated (no FXa) samples was calculated and data representing mean ± s.d. of three replicates is shown (unpaired, two-tailed t-test). The experiment was repeated three times