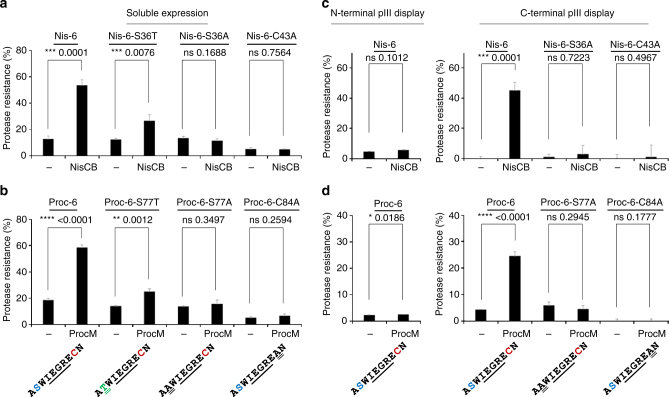
Fig. 2.
Assessment of the cyclization status of artificial lanthipeptides in cell lysates and displayed on phage. a Precursor peptides (full sequences in Supplementary Table 1) containing the NisA leader and indicated core sequences (residues involved in thioether formation colored) flanked by affinity tags were expressed with or without NisBC, captured from cell lysates, and subjected to FXa digestion and ELISA detection. The protease resistance relative to untreated (no FXa) samples was calculated and data representing mean ± s.d. of three independent cultures analyzed in duplicate is shown (unpaired, two-tailed t-test). b As in a, but core sequences were fused to the ProcA leader (full sequences in Supplementary Table 2) and expressed with or without ProcM enzyme. c Peptides containing the NisA leader sequence were translationally fused to the N- (left panel) or C-terminus (right panel) of phage pIII (full sequences in Supplementary Table 3), phage produced with or without NisBC co-expression, and the peptide cyclization status assessed on phage particles as described in a. d As in c, but phage displayed peptides containing ProcA leader sequences (full sequences in Supplementary Table 4) were tested after phage production with or without ProcM co-expression. Experiments shown in a–d were repeated three times