Abstract
Study Design
Systematic review.
Introduction
Prolonged hand edema can have detrimental effects on range of motion and function. There is no consensus on how best to manage traumatic subacute edema. This is the first systematic review which examines the clinical effectiveness of edema treatments on hand volume.
Purpose of the Study
The purpose of this systematic review was to examine the evidence of effectiveness of treatments for sub-acute hand edema.
Methods
A literature search of AMED, CINAHL, Embase, and OVID MEDLINE (from inception to August 2015) was undertaken. Studies were selected if they met the following inclusion criteria: randomized controlled or controlled trials in adults who have subacute swelling after a recent upper limb musculoskeletal trauma or cerebral vascular attack or after surgery. Two independent assessors rated study quality and risk of bias using the 24-point MacDermid Structured Effectiveness Quality Evaluation Scale (SEQES).
Results
Ten studies met the inclusion criteria. Study quality ranged from 23 to 41 out of 48 points on the SEQES. A total of 16 edema interventions were evaluated across the studies. Due to heterogeneity of the patient characteristics, interventions, and outcomes assessed, it was not possible to pool the results from all studies. Therefore, a narrative best evidence synthesis was undertaken. There is low to moderate quality evidence with limited confidence in the effect estimate to support the use of manual edema mobilization methods in conjunction with standard therapy to reduce problematic hand edema.
Conclusion
Manual edema mobilization techniques should be considered in conjunction with conventional therapies, in cases of excessive edema or when the edema has not responded to conventional treatment alone; however, manual edema mobilization is not advocated as a routine intervention.
Level of Evidence
2b.
Keywords: Edema, Compression, Hand therapy, hand, Treatment, Kinesiology tape
Introduction
The management of edema is a constant challenge for hand therapists where the objective is to reduce swelling as effectively and quickly as possible to focus therapy on more functionally related goals, such as return to usual activity. “Edema is glue”1 highlights the challenges of balancing the physiological healing process after injury with the need to maintain and restore soft tissue length, function and joint motion.
Prolonged swelling has an impact on joint range of motion, soft tissue mobility, quality of scar tissue formation, function, strength, and esthetics of the hand. These factors may delay a patient's recovery, return to work and resumption of activities of daily living and require frequent or increased outpatient appointments. Hunter and Mackin2 advocate a comprehensive therapy program to manage edema tailored to the individual needs of the patient and comprising a combination of evidenced-based interventions. “The prevention and treatment of edema are of paramount importance during all phases of management of the injured hand.”2
The most commonly used conventional treatment techniques in this phase include massage, elevation, exercise, and compression. Compression for hand edema is usually achieved through Lycra gloves which exert around 35 ± 5 mmHg pressure on the tissues of the hand.3 The garment acts as an external counter pressure4 which compensates for the inelasticity of edematous tissues, and, therefore improves circulatory efficiency by facilitating venous and lymphatic flow.3
Elevation permits gravity to assist with the drainage of edema from the distal limb.5 Elevation alone6 is not effective in reducing edema, but is recommended in combination with other modalities. Massage involves a “retrograde” action traditionally done in a distal to proximal direction. This technique uses a moderate force “milking” action but is considered too aggressive for the delicate lymphatic system to cope with and has recently been questioned.5
Recent evidence suggests that massage needs to be much lighter with only minimal pressure to traction the skin.7 It should start and end proximally to clear lymph channels proximally and make way for fluid distributed distally. This technique referred to as manual edema mobilization (MEM)7 is complimented with other methods aimed to assist with the facilitated direction of lymphatic flow which include low-stretch bandaging and a home exercise program. MEM massage does not involve pressure and in effect is more of a stroking action where the hand is brushed across the skin with only enough force to gently drag on the skin to the point at which it creases. Evidence suggests that a pressure of less than 30 mm Hg is sufficient, greater than 60 mmHg can cause damage to the lymphatics,8, 9 and at 75 mmHg, single cell lymphatic capillaries are completely collapsed.9
Active exercises which enable tendon gliding and muscular contractions can act as a pump which will assist with the flow of edema away from the periphery. Exercises can be completed in conjunction with other techniques to maximize the benefit; however, in certain circumstances, depending on the nature of the injury and/or surgery, the patients' hand movements are restricted based on healing timeframes and, if unable to use other techniques, this immobilization or restricted movement phase can have a detrimental effect of edema control.
Many of the advances in the management of edema after trauma are based on the research completed on lymphedema. Manual edema mobilization (MEM), which was introduced in 1995 as a method to reduce subacute and chronic hand and arm edema, has been adapted from the principles of manual lymph drainage (MLD) which is used to treat postcancer lymphedema.10, 11
MEM, according to Artzberger,10 consists of massage in a proximal to distal then distal to proximal direction, exercises, pump point stimulation, a home exercise program, and low-stretch bandaging. Kinesiology tape and myofascial release can also be used where necessary as a tissue softening method. In current practice, potential issues arise with interchanging terminology and a lack of awareness of the differences between the components of each technique.
Kinesiology tape, which can be used as part of the MEM program but also as an adjunct to the more traditional techniques, is designed to mimic the elastic properties of the skin by lifting the skin to allow greater interstitial space and encourage lymphatic drainage. In contrast to the traditional compression method which, using a glove and/or retrograde massage, is designed to push the fluid proximally into the venous and lymphatic system.12 The tape is said to be unique in that it mimics the elastic properties of the skin and its wave-like grain provides a pulling force to the skin creating more space by lifting the fascia and soft tissues under the areas where it is applied.13
The benefit of using it in the hand, unlike an edema glove, is that it leaves most of the skin surface free for sensory feedback which is essential for functional use. It can also be worn in water. As the tape is elastic and stretches up to 55%-60% of its length it also allows for unrestricted movement.13, 14 Kinesiology tape is becoming more popular for hand edema management and is already widely used in National Health Service clinical practice; however, there is no research evidence to suggest that it is effective in treating edema14, 15, 16 in the hand and there is limited understanding of its mechanism of action.17 As with some of the previous techniques mentioned, most of the research on kinesiology tape is also focused on its use in lymphedema18 where it has been shown to be effective. Given that lymphedema is a permanent and irreversible overloading of the lymphatic system, it is plausible that its mechanism of action may be similar to subacute edema where there is only a temporary overloading of the lymphatic system.
Purpose of the study
The purpose of this systematic review was to examine the evidence of effectiveness of a range of hand edema treatments on hand volume.
Methods
We conducted a systematic review using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) recommendations (http://www.prisma-statement.org/index.htm).19 The review protocol was registered prospectively on PROSPERO (CRD42015026836) http://www.crd.york.ac.uk/PROSPERO.
The following electronic databases were searched: the Cochrane Library (Wiley InterScience), MEDLINE (via Ovid), Embase (via Ovid), AMED (via Ovid), CINAHL (via EBSCO), SPORTDiscus (via EBSCO), PEDro (Physiotherapy Evidence Database), Allied Health Evidence, trial registers: Cochrane (Central Register of Controlled Trials) [CENTRAL] and the WHO (International Clinical Trials Registry Platform) from inception to August 2015. Search terms included: *EDEMA THERAPY/, exp EDEMA/TH [TH=Therapy], (hand ADJ edema).ti,ab, (edematous ADJ hand).ti,ab, *CRYOTHERAPY/, *RADIUS FRACTURES/, *FINGERS/, *HAND/, *WRIST/ OR *WRIST JOINT/, [Limit to: (Language English) and (Age group Adult) and Humans].
Additional references were searched for by examining the reference list of retrieved studies.
Eligibility criteria
Criteria for inclusion were English language, randomized controlled trails (RCTs) or controlled trials of adult participants with subacute swelling, after a recent upper limb musculoskeletal, hemiplegic stroke, or after surgery (ie, orthopedic and plastic). Active treatment must have occurred during the subacute phase. Subacute refers to swelling which is present after the initial acute inflammatory phase of ∼3-5 days and which persists into the fibroblastic phase between 2 and 6 weeks after trauma. Outcomes had to be assessed using a clinician derived measure of volume.
Studies were excluded if they used animals or humans where edema was investigated at an organ or cellular level. Studies using participants where edema was due exclusively to pregnancy or which only measure acute edema (day 0-14 after surgery or trauma) or chronic edema (around 3 months after surgery or trauma) were also excluded. Studies which only used a medicinal product or invasive methods to treat the edema (such as cortisone injection and anti-inflammatory drugs) were also excluded.
Data extraction
Extracting data from the included studies was done by the lead author (L.M.) using a purpose-designed standardized data extraction form. This form summarized details on study design, sample, interventions, outcomes, and results. On occasions when there was doubt over the interpretation of the data being extracted, a second reviewer (C.J.H.) also completed the data extraction independently using the same form to verify understanding and clarity of extracted data.
Assessment of methodological quality
Each included study was assessed for quality using the guidelines developed by MacDermid in the Structured Effectiveness Quality Evaluation Scale (SEQES).20 The scale consists of 24 items covering study question, design, subjects, interventions, outcomes, analysis, and recommendations and uses a 0-2 ordinal rating scale with 48 points maximum. A score of 2 means that the criterion was fully met, 1 = partially met, and 0 = criterion not met. To assess for risk of bias, 2 blinded reviewers independently rated each study in accordance with the evaluation guidelines recommended by MacDermid.20 This 24-item checklist covers 7 key components of risk of bias including adequacy of randomization and concealment of allocation, blinding of patients, health care providers and outcome assessors, extent of loss to follow-up, and analysis. Each of the 24 items has detailed descriptors, and scores can be summed into an overall score of methodological quality. Any disagreements between the reviewers were resolved by discussion.
The strength of the body of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines21 which assesses the risk of bias, publication bias, imprecision (random error), inconsistency, and indirectness. This final score is based on scores from 4 categories of evidence: quality, consistency, directness, and effect size and indicates the level of confidence in the effect estimate: high = at least 4 points overall, moderate = 3 points, low = 2 points, and very low = one point or less. Low and very low categories can be combined and were done so in this systematic review.
The 10 studies were grouped according to patient population: patients with subacute edema as a result of a musculoskeletal trauma or surgery and patients with subacute edema as a result of a hemiplegic stroke. This formed the basis of how results were analyzed and reported in this systematic review.
Results
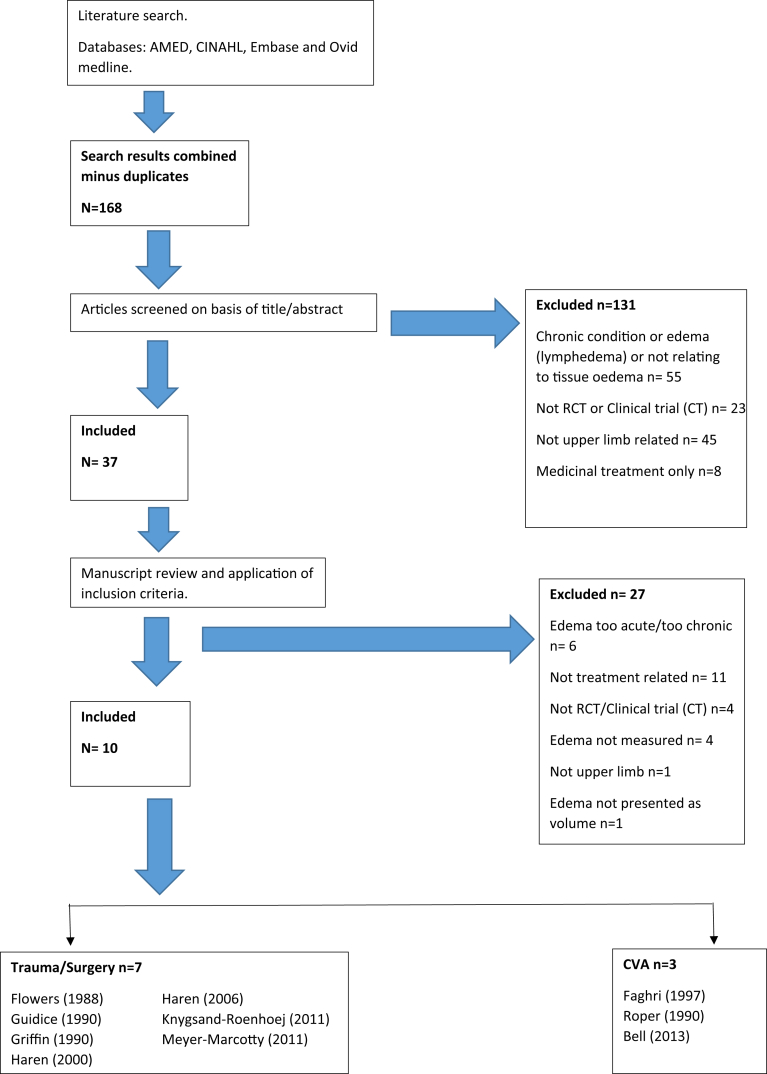
The initial search identified 168 articles for which titles and abstracts were screened. A total of 10 studies met the inclusion criteria (see PRISMA flow diagram in Figure 1) and were included in the review.
Figure 1.
PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analysis; RCT = randomized controlled trial; CT = clinical trial.
Quality scores ranged from 23 to 41 points out of 48 on the SEQES20 and 0-3 on using the GRADE checklist.21 Refer to Table 1 for quality assessment scores in rank order from the highest to lowest. The study characteristics of all eleven studies are summarized in Table 2.
Table 1.
Quality assessment scores (SEQES and GRADE)
| Patient pathology/author | Study question | Study design | Subjects | Intervention | Outcomes | Analysis | Recommendations | Total (48) | GRADE score | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | (4) | ||
| Trauma/surgery | ||||||||||||||||||||||||||
| Knygsand-Roenhoej (2011) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 41 | 3 |
| Haren (2006) | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 2 | 1 | 0 | 1 | 2 | 1 | 2 | 34 | 2 |
| Griffin (1990) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 2 | 29 | 1 |
| Haren (2000) | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 2 | 28 | 1 |
| Meyer-Marcotty (2011) | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 0 | 0 | 1 | 27 | 1 |
| Guidice (1990) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 2 | 26 | 1 |
| Flowers (1988) | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 23 | 0 |
| CVA | ||||||||||||||||||||||||||
| Faghri (1997) | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 0 | 1 | 2 | 0 | 1 | 2 | 1 | 2 | 30 | 1 |
| Roper (1999) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 29 | 1 |
| Bell (2013) | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 26 | 0 |
| Study question |
| 1. Was the relevant background work cited to establish a foundation for the research question? |
| Study design |
| 2. Was a comparison group used? |
| 3. Was patient status at more than 1 time point considered? |
| 4. Was data collection performed prospectively? |
| 5. Were patients randomized to groups? |
| 6. Were patients blinded to the extent possibly? |
| 7. Were treatment providers blinded to extent possible? |
| 8. Was an independent evaluator used to administer outcome measures? |
| Subjects |
| 9. Did sampling procedures minimize sample/selection biases? |
| 10. Were inclusion/exclusion criteria defined? |
| 11. Was an appropriate enrollment obtained? |
| 12. Was appropriate retention/follow-up obtained? |
| Intervention |
| 13. Was the intervention applied according to established principles? |
| 14. Were biases due to the treatment provider minimized (ie, attention and training)? |
| 15. Was the intervention compared with the appropriate comparator? |
| Outcomes |
| 16. Was an appropriate primary outcome defined? |
| 17. Were appropriate secondary outcomes considered? |
| 18. Was an appropriate follow-up period incorporated? |
| Analysis |
| 19. Was an appropriate statistical test(s) performed to indicate differences related to the intervention? |
| 20. Was it established that the study had significant power to identify treatment effects? |
| 21. Was the size and significance of the effects reported? |
| 22. Were missing data accounted for and considered in analyses? |
| 23. Were clinical and practical significance considered in interpreting results? |
| Recommendation |
| 24. Were the conclusion/clinical recommendations supported by the study objectives, analysis, and results? |
| Total quality score (sum of above/48) |
CVA = cerebral vascular attack; GRADE = Grading of Recommendations Assessment, Development and Evaluation.
GRADE score: high = 4/4, moderate 3/4, low 0-2/4.
Arranged in pathology subheadings and score from highest to lowest.
Table 2.
Summary of studies
| Author/date | Study design | Patients | Outcomes measured | Experimental intervention | Control | Timing of follow-up | Results | Conclusion | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Trauma/surgery | ||||||||||
| Knygsand-Roenhoej (2011) | RCT | Patients with unilateral postdistal radius fracture, treated with POP/internal or external fixation with subacute edema 4-10 wk after trauma/surgery and with a 60 mL+ in volume difference between the upper extremities (n = 30) | 1). Volumeter. standardized volumeter protocol recommended by the ASHT with 2 modifications: water temperature 23°-24° and patients were standing 2). AROM-PV distance (average of D2-D5) and thumb opposition 3). Pain using VAS 4). ADLs using custom designed questionnaire of bilateral activities and structured interviews 5). Perceived performance and satisfaction using the COPM (2+ change = clinically important) |
Isotoner glove (25-35 mmHg pressure) full time (except for hygiene and massage), regular therapy: ROM/strengthening HEP MEM: deep diaphragmatic breathing, exercises (proximal to distal), terminus stimulation, axillary stimulation (uninvolved side first), MPP stimulation to involved upper extremity, light skin traction in “U” shape massage, low stretch bandage system (if needed), exercising, and exercising during massage |
Elevation Compression: Coban (digits to proximal wrist) Functional retraining solitaire in elevation for 10 min + regular therapy (ROM/strengthening) + Flowtron intermittent compression system for 20 min. Isotoner glove (open fingers) night only (25-35 mmHg pressure) |
1,3,6,9, and 26 wk after inclusion in study. | Pretreatment modified MEM group (n = 14) mean (95% CI) 86.8 (73.0-100.6) Control group (n = 15) 96.3 (83.0-109.7) |
Posttreatment (9 wk) modified MEM group: 12.1 (0.2-24.1) Control group 28.3 (16.8-39.8) |
Tendency for MEM group to receive 20% fewer OT session (edema and other treatments) than the control group, however not SS (P = .13) Either approach is satisfactory (statistically significant difference in edema reduction between inclusion and the last follow-up in both groups), however, as the MEM group had fewer sessions, this is recommended for subacute edema |
|
| Haren (2006) | RCT | Patients with distal radius fracture treated with plaster or external fixation with edema of hand and wrist of more than 40 mL difference between volume of uninjured and injured hand (using volumeter) (n = 51) | Volumeter with water heated to room temperature. Uninjured hand measured first. Hand dominance estimated to be 3.43% larger than nondom hand according to standard techniques. All other edema measurements were made on injured hand and compared to pretreatment volume of injured hand. | First 6 treatments included 40 min of MLD in additional to conventional treatment of elevation, active and resistive exercises (hand and wrist), and compression (edema glove- night and day until first measurement) Verbal and written instructions (HEP) Encouraged to use hand as much as possible. |
Conventional treatment of: elevation, active and resistive exercises (hand and wrist), and compression (edema glove- night and day until first measurement) Verbal and written instructions (HEP) Encouraged to use hand as much as possible. |
Second measurement 60 d after inclusion (49-71) for experimental group and 56 d (32-63) after inclusion for control group | Pretreatment experimental median normal size before trauma 545 mL (95% CI: 372-595) Control median normal size before trauma 453 mL (95% CI: 343-637) |
Posttreatment experimental first measurement median decrease in injured hand 30 mL (95% CI: 10-55) Control median decrease in injured hand: 20 mL (95% CI: −10 to 45) Second measurement experimental median decrease in injured hand: 40 mL (95% CI: 10-90) Control median decrease in injured hand 35 mL (95% CI: 15-80) Statistically significant difference in edema reduction with a large overall reduction in the experimental group at the first measurement (P = .005) At second measurement, a greater reduction was seen in the experimental group, but this was not statistically significant. |
Study supports the use of MLD as complimentary to conventional therapy when there is excessive edema | |
| Griffin (1990) | RCT | Patients with trauma to 1 upper extremity at least 2/52 before study participation and with clinically significant (visually detectable swelling of sufficient magnitude to be considered a problem) hand edema judged by 1 PT. (n = 30) | Volumeter (mL) measured in affected and unaffected sides before rest. Ten-min rest with the arm at heart level and patient seated. In second measurement, 30-min treatment, then third volumetric measurement of affected hand. | High-voltage pulsed current (HVPC); n = 10. One electrode over MN, other over UN, and dispersive electrode over dorsolumbar region of back. Intensity adjusted to produce observable and maintainable muscle contracture of FPL/FPB and dorsal lumbricals, 8 (twin) pulses per second alternating between 5 s of UN and 5 s of MN. | In placebo HVPC, dispersive electrode was disconnected without the subject's knowledge | Postrest (10 min) and posttreatment (30 min) measurements | Before rest: Placebo HVPC: In unaffected hand: 512.2 (SD = 104.1) And in affected hand: 573.1 (SD = 111.2) HVPC: In unaffected hand: 507.3 (SD = 54.2) and in affected hand: 553.7 (SD = 75.0) IPC: In unaffected hand: 503.8 (SD = 82.9) And in affected hand 557.4 (SD = 92.4) |
After rest: Placebo HVPC: 572.1 (SD = 109.9) HVPC: 553.3 (SD = 73.8) IPC: 558.4 (SD = 92.1) |
After Rx: Placebo HVPC: 570.8 (SD = 109.5) HVPC: 547.0 (SD = 73.0) IPC: 550.7 (SD = 92.1) |
No change occurred after rest period therefore concluded that patient activity before session did not affect measurement. Wide variability in HVPC and IPC in amount of posttreatment change 0-15 mL hypothesis rejected. Prerest and postrest hand volumes in 30 subjects are not significantly different (Wilcoxon test P = .761) Mean change between before and after rest= 0.13 mL (−3 to 8) Posttreatment volume: KW test significant difference between IPC, placebo and HVPC groups (P = .011) Wilcoxon rank-sum significant difference between IPC and placebo (P = .004) No significant difference between placebo and HVPC (P = .446) Difference between HVPC and placebo HVPC did not reach statistical significance (P = .036) |
| Haren (2000) | RCT | Patients with distal radius fractures requiring an external fixator (n = 26) | Volumeter (4 measurements) difference in volume calculated in mL between uninjured and injured. Water of room temperature | Ten MLD treatments: light surface massage proximal to distal + elevation, active, passive exercises, and compression with elastic bandages (Elastomull) during ex-fix period then tubigrip or isotoner glove after removal of ex-fix. The use of hand encouraged as much as possible, verbal instructions and written program for HEP. | Elevation, active, passive exercises, and compression with elastic bandages (Elastomull) during ex-fix period then tubigrip or isotoner glove after removal of ex-fix. The use of hand encouraged as much as possible, verbal instructions and written programme for HEP. | 3, 17, 33, and 68 d after removal of external fixator | Experimental group mean (SD) differences between volume measures (mL) of injured and uninjured hand in d 3: 39 (SD = 12); d 17: 27 (SD = 9); d 33: 19 (SD = 9); d 68: 12 (SD = 11) 95% CI mean differences between groups in d 3: 0.6-49.5; d 17: 2.2-43.4; d 33: −0.3 to 31.5; d 68: −1.0 to 24.2 A significant difference in hand volume, with a lesser degree of edema in the group treated with MLD, was recorded at the first 2 measurements. Probability at the first measurement was P = .04 (n = 26) , second measurement was P = .1, and at fourth measurement was P = .2 |
Control group mean (SD) differences between volume measures (mL) of injured and uninjured hand in d 3: 64 (SD = 41); d 17: 50 (SD = 35); d 33; 35 (SD = 26); d 68: 24 (SD = 20) | Edema treatment should be initiated during early fracture healing Patients in MLD group will have less edema at an earlier posttraumatic stage compared with conventional treatment, which reduces risks of edema-associated complications. MLD not proposed for all patients with hand edema after #DR but as complementary to conventional treatment when edema is troublesome |
|
| Meyer-Marcotty (2011) | RCT | Patients undergoing elective wrist arthroscopy for TFCC lesions, intracarpal ligament ruptures, and/or damage to the wrist cartilage (n = 54) | 1). Pain VAS (0-10) + pain diary 2). ROM (extension, flexion, radial and ulnar deviation, and pro/supination) using goniometer. Overall global ROM = summation of 3 different directions of motion measured from dorsum of wrist. 3). Water displacement with volumeter. Displaced water collected and expressed in mL. Water temperature 28°. 4). DASH 0-100 score. |
10 min of cooling-compression period before sterile of arm. Cryo-Cuff applied to operated wrist. 30 mmHg pressure. 3 × 10 min for 22 d (at least twice daily) | Apply cryotherapy of either mode (cool packs or crushed ice) wrapped in a towel to operated wrists. No interval or frequency given just PRN. | D 1, 8, and 21 after arthroscopy | Volume of wrist and forearm tended to be lower in experimental group from preop to d 1: 967 ± 24 to 932 ± 34 mL (not SS) The control group had slight but not significant increase in volume: 890 ± 36 to 912 ± 38 mL Volume unchanged from preop to d 21 (not SS): in experimental: 967 ± 24 vs 954 ± 25 mL and in control: 890 ± 36 vs 905 ± 33 mL |
No difference between both study groups in terms of volume change over time. No significant effect on hand volume, pain, ROM or DASH scores between groups over a 3-wk period. |
||
| Guidice (1990) | Crossover trial | Patients with upper extremity injury/surgery more than 4 wk ago or 4/52 after onset of upper extremity paresis (n = 16) | 1). Circumferential measures (mm) of proximal phalanx of most visibly edematous finger 2). Finger stiffness determined by PROM of MCPJ flexion using goniometer and 200 g constant force gauge applied for 5 s 3). Volumeter (mL), average of 2 successive volumetric measures of affected hand |
Elevation and 30 min of continual passive motion. Extension and flexion of D2-5 Wrists supported with universal wrist splint provided with CPM machine during treatment |
Elevation alone (30 min) supine on flat surface, limb maintained on stand at 30° shoulder abduction, 30° shoulder flexion, and 70° elbow flexion. Wrists supported with universal wrist splint provided with CPM machine during treatment | Immediately after treatment | Elevation alone: Change score (SD)/% change (SD): 1) 0.6 mm (0.6)/0.8 mm (0.8) 2) 6.1 mL (9.5)/1.1 mL (1.8) CPM with elevation resulted in a significantly greater reduction of hand edema than elevation alone. Sequence effects were not significant for measures of hand volume and finger circumference. Small-to-moderate (0.2 and 0.3) +ve relationship (between treatment outcome and time after onset) for reduction in hand volume following elevation alone. Almost no relationship was found for hand volume and finger circumference following CPM with elevation or finger circumference following elevation alone. Moderate-to-large +ve relationship (0.4 and 0.5) (between treatment effect and amount of pretreatment edema) for hand volume and finger circumference with CPM and elevation |
CPM with elevation: Change score (SD)/% change (SD): 1) 1.4 mm (0.9)/1.9 mL (1.2) 2) 14.5 mL (8.4)/27 mL (1.6) |
Measures of edema that were reduced following CPM and elevation generally returned to pretreatment level within 24 h. The greater the time after onset the greater treatment effect. The greater the amount of pretreatment edema, the greater the treatment effect. 30 min of CPM with limb elevation resulted in a significantly greater reduction in hand edema than 30 min of elevation alone. Findings for total group similar to subgroup analysis of CVA (N = 11) group suggest that CPM with elevation is an effective treatment to reduce hand edema for patients with hemiplegia after CVA |
|
| Flowers (1988) | Crossover trial | Patients with generalized hand edema due to hand or wrist injury, surgery, pregnancy, or venous stenosis (n = 14) | Circumferential measurement at the middle level of the PIPJ using a Jobst tape measure. PIPJs were marked with a fine-tip pen before each treatment. Proximal edge of tape measure placed over pen mark. PIPJs held in comfortable end of range extension | A). Traditional retrograde massage: Stroke distal to proximal over entire length of affected digit with a firm milking action using baby powder as lubricant. Continuous strokes for 5 min B). String wrapping: Coiling #36 ball twine around digit from nail bed to web space. Each successive loop placed directly next to preceding loop with no gaps for 5 min. Snug but not tight C). String wrapping with continuous superimposed retrograde massage: Apply string wrapping as in (B) with (A) performed over the string for 5 min D). String wrapping with intermittent superimposed retrograde massage: Massaging the string wrapped digit for 20 strokes. String wrapping removed rapidly and reapplied immediately and followed by another 20 strokes for 5 min |
Immediately after treatment | Average circumferential reductions (%) A) Retrograde massage: 1.35% B) String wrapping: 1.74% C) Continuous massage with string-wrapped digits for 5 min: 3.46% D) Intermittent massage of string wrapped digit for 5 min: 2.95% No significant difference between string wrapping and retrograde massage. ANOVA showed a significant difference existed between treatments (P ≤ .001) Wilcoxon test significant differences between the 4 techniques, except between A and B. C>A (P = .01) D>B (P = .01) C>D (P = .05) First digit treated showed greatest circumferential reduction Order of digit treated had no significant bearing on outcome |
A combination of string wrapping with intermittent retrograde massage is consistently more effective in reducing circumferential edema in digits than either massage or wrapping alone | |||
| CVA | ||||||||||
| Faghri (1997) | CT | Patients with visible hand edema after CVA (less than 6/12 ago) (n = 8) | 1). Volumeter: Average of 3 successive measures (mL) of affected hand/forearm 2). Circumferential girth measures of upper and lower arms using flexible tape measure |
Neuromuscular stimulation + usual activities including treating edema. Frequency 35 Hz to create reciprocal activity of flexors and extensors of lower arm. Ten-second action of wrist and finger flexors, 10-s action of wrist and finger extensors, and 10-s rest. Total treatment time: 30 min | Elevation + usual activities including treating edema. Thirty minutes of elevation in a standardized position previously recommended by other investigators as most effective and comfortable: lay supine, 30° shoulder abduction, 30° shoulder flexion, and 70° elbow flexion. |
Immediately after treatment | Mean change scores: NMS: Hand volume (mL): −13.38 (SD = 2.03) Arm volume (mL): −32.63 (5.83) Lower arm girth (mm): −8.75 (1.26) Upper arm girth (mm): −7.50 (1.65) Elevation: Hand volume (mL): 1.88 (3.90) Arm volume (mL): 26.5 (9.81) Lower arm girth (mm): 1.30 (2.29) Upper arm girth (mm): 1.25 (2.29) |
% change scores: NMS: 2.64% (SD = 0.53) 1.97% (0.45) 3.88 (0.58) 2.63 (0.64) Elevation: 1.89 (0.67) 1.35 (0.51) 0.63 (0.95) 0.35 (0.77) |
In 8 subjects, 30 min of NMS is more effective than 30 min of elevation Measures of edema that were reduced following 30 mins of NMS returned to pretreatment levels within 24 h No carry over effect (sequences/d of treatment) for NMS/elevation |
|
| Roper (1990) | RCT | Patients with a first ever hemisphere stroke (WHO criteria) and edema of hemiparetic hand (>20 mL volume in stroke hand compared with unaffected hand after 2 readings, 1 wk apart) (n = 37) | 1). Volumeter (device made for study, not a standardized tool) average of 3 measurements taken from both hands. 2). Motricity Index |
Intermittent pneumatic compression + standard physiotherapy, 50 mmHg applied with a 30-s inflation and 20-second deflation cycle in 2 sessions of 2 h a day for 1 mo | Standard physiotherapy (pragmatic) included positioning and passive movements. | Weekly during a 4-wk treatment period | Pretreatment Mean volume (affected hand–unaffected hand): Experimental: 52.7 mL (SD = 27.2) Control: 63.7 mL (SD = 23.7) No change in experimental group in mean hand volume after treatment (P = 1.0) Nonstatistically significant decrease in mean hand volume of 3.2 mL (SD = 33.2) (P = .69) No statistically significant difference between the 2 groups (P < .65) T-test: treated group vs control group P = .59 |
Posttreatment mean volume (affected hand–unaffected hand) Experimental: 52.7 mL (SD = 36.9) Control: 60.5 mL (SD = 32.7) |
Standard physio had a non-SS decrease in edema Edema can resolve spontaneously (n = 17, not eligible) Parameters of the compression machine were inadequate. IPC cannot be recommended at this pressure/duration |
|
| Bell (2013) | RCT | Patients with hemiplegic stroke within the last 3/12 and presence of edema by visual inspection (n = 17) | 1). Circumferential measurements of wrist and MCPJs using spring loaded Gulick anthropometric measuring tape 2). Upper limb portion of Fugl-Meyer Assessment (FMA). Total 66 points (higher score = better function) |
Kinesiology tape with 20% stretch. Dorsal and volar application with buttonhole technique covering 2/3 of forearm for 6 d (replaced as/when needed) + standard OT, PT, and SLT. | Standard physical, occupational, and speech and language therapy. Including positioning, active, and passive range of motion. | 6 d after baseline. | Before treatment Experimental: Median MCPJ circumference (cm): 21.4 (SD = 2.0) Median wrist circumference (cm): 18.0 (SD = 1.7) Control: Median MCPJ circumference (cm): 20.7 (SD = 1.7) Median wrist circumference (cm): 17.8 (SD = 1.5) Experimental group showed a small reduction in MCP and wrist circumference measurements, greater results at MCPJ. Control group showed an increase in both areas. No statistical difference between the 2 groups for change at MCPJs (P = .111) or change at the wrist (P = .189) A large effect size was seen at the MCPJ (0.8) and a medium ES at the wrist (0.7) |
After treatment Experimental: Median MCPJ circumference (cm): 0.5 (SD = 0.65); −0.1 to 2.2 Median wrist circumference (cm): 0.2 (SD = 0.4); 0 to 1.1 Control: Median MCPJ circumference (cm): −0.3 (SD = 0.91) =1.0 to 1.6 Median wrist circumference (cm): −0.1 (SD = 0.57); −0.5 to 0.8 |
8/9 patients (88%) had edema reduced in experimental group: 1 patient had increased edema. Median negative change in control group indicated edema worsened over the 6-d trial. ES of KT is smaller than those reported with NMES, CPM, and Lycra garments; however, KT is cheaper and quicker to apply |
|
ANOVA = analysis of variance; AROM-PV = active range of motion-pulpa vola; ASHT = American Society of Hand Therapy; CI = confidence interval; COPM = Canadian occupational performance measure; CPM = continual passive motion; CT = controlled trial; CVA = cerebrovascular accident; DASH= Disability of the Arm, Shoulder and Hand; D/C = discharge; #DR = fractured distal radius; ES = effect size; FPB = flexor pollicis brevis; FPL = flexor pollicis longus; HEP = home exercise program; HVPC = high-voltage pulsed ultrasound; IPC = intermittent pneumatic compression; KT = Kinesiology tape; KW = Kruskal Wallis; MCPJ = metacarpal phalangeal joint; MCPs = metacarpal interphalangeal joints; MEM = manual edema mobilization; MLD = manual lymph drainage; MN = median nerve; MPP = manual pump point; NMS = neuromuscular stimulations; NMES = neuromuscular electrical stimulation; OT = occupational therapy; PIPJs = proximal interphalangeal joints; POP = plaster of Paris; PRN = per required need; PROM = passive range of motion; PT = physiotherapy; PV = pulpa vola distance; RCT = randomized controlled trial; ROM = range of motion; Rx = treatment; SD = standard deviation; SLT = speech and language therapy; SS = statistically significant; TFCC = triangular fibrocartilage complex; UN = ulnar nerve; VAS = visual analogue scale; WHO = World Health Organization.
Arranged in pathology subheadings and quality assessment score from highest to lowest.
Sample sizes ranged from 8 to 54 patients. There were a total of 361 participants across the 10 studies whose age ranged from 18 to 85 years.
A total of 16 interventions were described; these included kinesiology taping, massage (retrograde and intermittent), normal functional use, strengthening, MLD, MEM, elevation, high-voltage pulsed ultrasound, cryotherapy, neuromuscular stimulation (NMS), positioning/orthosis, active/passive exercises, and compression which was administered in numerous forms: string wrapping, isotoner glove, intermittent pneumatic, or Coban.
All studies used either circumferential measurements (in centimeters or millimeters) or volumetry (mL) to quantify volume.
Four studies22, 23, 24, 25 used the same method of analysis: mean volume of edema (mL), whereas some26, 27, 28 used percentage change (mL and mm), other authors used a variety of mean difference, median decrease, and median circumference.
Only 3 of the 10 studies examined similar interventions.22, 29, 30 They assessed the effectiveness of MLD/MEM vs standard treatment. Although the authors use different terminology, which in itself may not be an accurate representation of the techniques being used, they essentially comprised very similar techniques which include light massage (in a proximal to distal direction), some form of compression (low stretch bandages or a glove), elevation, exercises, and breathing techniques, hence why they have been grouped together during analysis.
These 3 studies also used the same outcome measure, the volumeter (mL), however used different methods of analysis (mean difference, median decrease, and mean volume) when expressing their outcomes. The combination of results for meta-analysis was not possible because of differences in the methods of reporting results or heterogeneity of interventions and outcomes assessed.
Interventions
Summary findings for individual studies are presented under 2 headings according to the patient type (trauma/surgery or cerebrovascular accident [CVA]) and listed from highest to lowest quality within these headings, as assessed by SEQES and GRADE scores. For full study details and results, refer to Table 2.
Evidence on edema techniques after trauma and surgery
MEM + conventional therapy vs conventional therapy22
Both groups had a statistically significant difference in edema reduction between inclusion in the study and penultimate follow-up (9 weeks); however, there was no statistically significant difference in any outcomes between the groups.22 In light of this, therefore, authors conclude that using conventional therapy with or without the addition of MEM is satisfactory in treating edema; however, as the MEM group had 20% fewer sessions (not statistically significant P = .13) than the control group who had conventional therapy alone, this is recommended for subacute edema. The interventions were well described with the use of 2 independent therapists performing the treatment and 2 blinded assessors conducting the outcome measures.
MLD + conventional therapy vs conventional therapy alone30
Both groups had a reduction in edema after treatment. A statistically significant difference in edema reduction was seen with a large overall reduction in the experimental group at the first measurement (P = .005).30 At the second measurement, a greater reduction was observed in the experimental group, but this was not statistically significant. The authors concluded that MLD should be used as complimentary to conventional therapy when there is excessive edema; however, there were limitations in this study such as post hoc subgroup analysis being performed, the lack of a secondary outcome, and failure to recruit their original target sample size of 82 patients. A sample size of 51 reduced the power from 90% to 73%; the confidence intervals were very wide indicating poor precision in their estimate, and therefore, their conclusion needed to be interpreted with caution.
High-voltage pulsed current (HVPC) vs intermittent pneumatic compression (IPC) vs placebo HVPC23
No significant difference was found between IPC and HVPC (P = .446), HVPC and placebo HVPC (P = .036), but a difference was seen between IPC and placebo HVPC (P = .004).23 Overall, IPC gave the best result, with a 2%-3% reduction in edema from postrest values, however, without the effect estimates which were not documented these P values tell us very little about the strength of the effect and its clinical significance. One of the major flaws with this study was the lack of independent treatment provider and assessors. The same physical therapist administered all treatments and conducted all measurements.
MLD + conventional therapy vs conventional therapy alone29
Although the experimental intervention is called MLD, only massage is described which makes the use of this term misleading.29 No inclusion or exclusion criteria were reported, no pretreatment measures were taken, and secondary outcomes were not considered. Inappropriate statistical testing was used which assumes a normal distribution (Kruskal-Wallis test instead of a Mann-Whitney test), and the lack of a sample size calculation reduces the reliability of the results and conclusions given. Patients in the MLD group were seen a mean of 3 additional times compared to the control group. The authors defend this as being necessary as they were adding MLD to conventional therapy and not trying to replace it, which may explain why they do not recommend MLD for all patients after fracture distal radius but as complementary to conventional treatment when edema is troublesome. The clinical significance is not adequately described.
Cooling compression vs cryotherapy24
There was no difference between groups in terms of volume change over time in this study.24 A lack of reported detail prevented adequate comparison of baseline patient characteristics. The authors state that blinding assessors was not possible in this trial, however, as all patients will have had arthroscopy scars and could have removed the Cryo-Cuff or ice pack before their follow-up assessments on day 1, 8, and 21 after arthroscopy this could have minimized bias and improved the validity of this study. A sample size calculation was done based on a 20% difference in pain levels (primary outcome measure); however, inadequate analysis was performed for the secondary outcomes (volume change) as no effect size, P value, or confidence intervals were reported, and therefore, we are unable to assess if the study was adequately powered to identify treatment effects for edema reduction.
Elevation and continual passive motion vs elevation alone27
Continuous passive motion with elevation resulted in a significantly greater reduction of hand edema than elevation alone.27 However, the reduction in edema in this group generally returned to pretreatment levels within 24 hours highlighting a limitation in their follow-up period. The use of an independent assessor was not reported, and a sampling or standardized enrollment was described with limited patient characteristics being made available, all of which could give rise to bias. This is the only study which had a mixed cohort of patients whose edema was from either a trauma/injury or paresis. Findings for the total group were similar to a subgroup analysis of the CVA group (n = 11) and although the authors suggests that continual passive motion and elevation is an effective treatment to reduce hand edema for patients with hemiplegia after CVA, the results do not support this given the very short term and reversible reduction in hand edema.
Retrograde massage vs string wrapping vs continuous massage and string wrapping vs intermittent massage and string wrapping26
This cross over trial failed to give adequate details on its study design and subjects and therefore scored the lowest in the quality assessments.26 Although the treatment order and digit to be treated was randomized, the time between visits was not documented meaning we are unable to assess if an adequate “wash-out” period was given between each of the treatments. The lack of an independent assessor meant that the same therapist performed the treatment and administered the outcome measures. The authors chose to do a post hoc analysis which demonstrated a statistically significant difference (t = 20, P = .05) between continuous and intermittent massage (along with string wrapping), but size and significance of the treatment effects were not reported.
Evidence on edema techniques after CVA
NMS and usual activities vs elevation and usual activities28
Limited details regarding the study design meant we were unable to determine who issued the treatments and whether the assessors were independent and blinded.28 Although patients were not randomized to groups in this repeated measures study, the patients acted as their own control by receiving both treatments, therefore reducing the need for large sample sizes and homogeneity in patient characteristics such as age and severity of CVA. However, although the size and significance of the effects were reported, it was not established if the study has sufficient power. Both groups were instructed to carry out their usual activities which included treating edema. No details were given on these “other” edema treatments and whether they were standardized across both arms of the trial, and therefore, we are unable to ascertain whether the reduction in edema was purely due to the NMS.
Kinesiology tape and standard occupational therapy (OT) and physiotherapy (PT) vs standard OT and PT31
No sample size calculation was performed in this study and, as only 17 of the enrolled 25 patients had complete data, this loss to follow may have given rise to a type II error.31 A qualified taping practitioner performed all taping on the patients, and 2 independent raters completed all outcome measures; however, there were no details on whether the treating therapists were blinded to the group allocation. To further reduce bias, a placebo or sham tape application could have been used in the control group, as despite using blinded raters they assessed the experimental group within 30 minutes of the tape being removed which, due to marks left on the skin, may have unblinded them.
Intermittent pneumatic compression and standard PT vs standard PT25
The main issues in this study relate to their analysis as it was not established that the study had significant power to identify treatment effects and the authors fail to give confidence intervals or effect sizes with the P values.25 Although randomization was performed, limited detail was given on the precise method. Possible baseline differences were not adjusted in their analysis as the mean time since the stroke was nearly twice as long in the control arm and this group had more pretreatment edema (t-test P = .59); this could be a meaningful difference which may have confounded the results.
Discussion
This is the first systematic review of its kind to review the evidence on the effectiveness of conservative treatments for subacute hand edema in patients after trauma, surgery, or stroke.
Methodological quality
The overall quality of the 10 studies was low to moderate with most studies scoring consistently poor marks on 4 particular questions on the SEQES20 relating to the lack of an independent evaluator to perform outcome measures, appropriate enrollment process, appropriateness of secondary outcomes, and sufficient power to identify treatment effects. Low scores were given when the study did not meet the criteria or where there was insufficient detail to make a judgment on that particular question.
Reporting quality
Poor reporting quality was a limitation of all the included studies. The level of detail recommended in the CONSORT 201032 statement's 25-point checklist was not adhered to by most of the included studies; however, 7 of the 10 studies were published before the Consolidated Standards of Reporting Trials (CONSORT) recommendations were introduced. This lack of transparency in the reporting affected our ability to adequately assess the validity of the results. In some cases, both the experimental and control interventions were not described in enough detail for them to be reproduced. The “black box” of rehabilitation is well documented33, 34, 35, 36 with the lack of reported detail on the components of treatments being one of the main methodological limitations of research studies in rehabilitation.
“Standard therapy/care”25 and “usual activities”28 were not described in sufficient detail to allow the reader to adequately understand the specific treatment that were being implemented or to differentiate between the experimental and control intervention.
Type I and II error
Seven of the 10 included studies did not document their sample size calculations so we were unable to establish if these studies had sufficient power to identify treatment effects. This may have increased the likelihood of type II errors occurring. The lack of randomization28 and issues with blinding and/or independent assessors could also have given rise to type I errors.24, 26, 27, 28
Heterogeneity of patients
Variations in patient characteristics within and between the 10 studies may have influenced the treatment effects and was one factor which limited comparisons as no stratification or subgroup analysis had been completed. Flowers26 included pregnant women alongside patients with venous stenosis and after hand/wrist surgery. The differing etiology indicates that conditions such as water retention during pregnancy may be temporary, transient, and fluctuating, whereas patients with venous stenosis may have this condition due to a chronic thickening of the blood vessels secondary to trauma or external compression of the musculoskeletal system and that this may require surgical or pharmacological interventions.
Haren and Wilberg30 included patients with external fixator 3-5 days after it had been removed. Patients with external fixators were left a mean of 47 days (experimental group) and 43 days (control group), whereas the external fixator was in place with no edema management in place. Patients treated with external fixators had this fixation on for an average of 13 days longer than those patients treated with plaster of Paris which meant the time from fracture to treatment start date was delayed. Although there was an equal distribution of plaster to external fixators in both groups, patients with external fixators may have had more, longer standing, and untreated edema which could have impacted on the success of the intervention.
Variations in interventions
Inconsistencies in how the same modality was delivered across studies, along with the issue of a lack of reported detail prevented appropriate comparison. Haren et al29 and Flowers26 both used massage as part of their experimental intervention; however, Flowers26 used a one off 5-minute treatment, whereas Haren et al29 used 10 sessions, but did not comment on the duration. In the study by Meyer-Marcotty,24 the control group used cryotherapy either with cool packs or crushed ice to operated wrists. However, unlike the structured experimental group who were instructed to apply the Cryo-Cuff twice daily for 10 minutes, the control group had no stipulated frequency or duration. Although this “Per Required Need” approach may reflect real life, for the purposes of the research it would have been useful to document the control group usage of cryotherapy to establish the effect of adding the regular compression element in the intervention group.
The details that were given for the interventions highlighted conflicting theories particularly relating to massage. Flowers26 describes a “firm milking action” in a distal to proximal direction, whereas Haren et al29 uses a “light surface massage” in a proximal to distal direction and Knygsand-Roenhoej and Maribo22 complete “light traction massage” in a ‘U’ shape from proximal to distal. This difference may be due to advances in clinical practice since the 1980s when Flowers conducted his study and while “retrograde massage” is still used in clinical practice it has been adapted to a lighter action as opposed to a firm milking one which is thought to be too aggressive on the delicate lymphatic system.37
The interventions described by Haren et al29, 30 as MLD and Knygsand-Roenhoej22 as modified MEM constituted a set of very similar techniques, however because of disparity in quality assessment scores, particularly between the 2 studies by Haren et al29, 30 they could not be analyzed together. Consistent terminology is required to avoid confusion and to ensure understanding of the interventions.
Length of follow-up
Follow-up ranged from immediately after treatment to day 68 after treatment (∼9 weeks). Four of the 10 studies assessed edema immediately after the intervention,23, 26, 28, 29 and although some showed a statistically significant reduction in edema, this returned to pretreatment levels within 24 hours indicating that a longer term follow-up was required to see if the effects of the intervention have been maintained over time.
Strengths and limitations of the review
The strengths of the review include the specific inclusion criteria, the adherence to the PRISMA recommendations,38 and a priori protocol publication on the PROSPERO Web site; however, this review also had a number of limitations. First, due to the lack of RCTs and controlled trials of edema management techniques in this specified population, older studies were incorporated with more recent ones using more current interventions, and therefore, comparison between techniques which have changed over time may indicate a limitation of including studies of any age in this systematic review. Despite the lack of comparability of included studies, this review serves as a baseline on this topic and a start point for future studies to improve on.
Conclusions
The review found limited low-to-moderate quality evidence to support the use of a combination of interventions known as MEM when treating problematic subacute hand edema compared with standard treatment alone. The results need to be interpreted with caution due to numerous limitations associated with the quality assessment of the included studies. Due to the number of different modalities used across the studies, there was little consensus in the literature of the most appropriate methods, dose response, and duration of even the “standard” interventions. Although every patient will require a tailored approach to edema management, clinical guidelines based on evidence from high-quality RCTs may aid the choice and delivery of techniques to improve effectiveness.
The clinical implications arising from the current evidence synthesis are based on the 2 studies with the highest (moderate) quality.22, 30 Therapists should continue to use a combination of conventional interventions which include elevation, exercise, and compression to manage subacute hand edema after trauma. MEM techniques should be considered, (if not medically contraindicated) in conjunction with conventional therapies, in cases of excessive edema or when edema has not responded to conventional treatment alone. Using the MEM method, in addition to conventional treatment, may reduce the number of sessions required.22 The MEM technique used by Haren and Wilberg30 is not described, and therefore, readers are required to refer to their earlier article29 which gives more detail. However, it does not fully describe the correct MEM technique and is referred by the authors as MLD which is an inaccurate terminology.
Further high-quality RCTs are needed to assess the effectiveness of therapy interventions on hand volume for subacute hand edema, particularly focusing on the methods of delivery and application, instructions to patients, dosage, and duration for a range of edema treatments.
Acknowledgments
Leanne Miller is funded by a National Institute for Health Research and Health Education England Clinical Doctoral Research Fellowship. Christina Jerosch-Herold is funded by a National Institute for Health Research Senior Research Fellowship.This article presents independent research funded by the National Institute for Health Research (NIHR) and Health Education England. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Footnotes
Conflict of interest: All named authors hereby declare that they have no conflicts of interest to disclose.
JHT Read for Credit
Quiz: #504
Record your answers on the Return Answer Form found on the tear-out coupon at the back of this issue or to complete online and use a credit card, go to JHTReadforCredit.com. There is only one best answer for each question.
-
#1.The study design is
-
a.RCTs
-
b.prospective cohort
-
c.systematic review
-
d.a case series
-
a.
-
#2.The number of studies that met the inclusionary criteria is
-
a.11
-
b.15
-
c.21
-
d.39
-
a.
-
#3.The most common traditional interventions include
-
a.compression
-
b.elevation
-
c.massage
-
d.all of the above
-
a.
-
#4.MEM theoretically is intended to activate the
-
a.venous return system
-
b.arterial pumping system
-
c.lymphatic system
-
d.none of the above
-
a.
-
#5.There is compelling evidence to suggest the efficacy of manual edema mobilization
-
a.true
-
b.false
-
a.
When submitting to the HTCC for re-certification, please batch your JHT RFC certificates in groups of 3 or more to get full credit.
References
- 1.Watson-Jones R. 4th ed. E. & S. Livingstone Ltd; Edinburgh and London: 1955. Fractures and Joint Injuries. [Google Scholar]
- 2.Hunter J.M., Mackin E.J. Edema: techniques of evaluation and management. In: Hunter J.M., Mackin E.J., Callahan A.D., editors. Rehabilitation of the Hand: Surgery and Therapy. 4th ed. Mosby Year Book; Baltimore: 1995. pp. 77–81. [Google Scholar]
- 3.Mann R., Yeong E.K., Moore M., Colescott D., Engrav L.H. Do custom-fitted pressure garments provide adequate pressure. J Burn Care Rehabil. 1997;18:247–249. doi: 10.1097/00004630-199705000-00013. Marieb EN, Hoehn K (2007) Human Anatomy. [DOI] [PubMed] [Google Scholar]
- 4.Zuther E.Z. 2nd ed. Thieme; New York, NY: 2009. Lymphedema Management. [Google Scholar]
- 5.Villeco J. Edema: A silent but important factor. J Hand Ther. 2012;25:153–162. doi: 10.1016/j.jht.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Fagen D.J. A controlled clinical trial of postoperative hand elevation at home following day-case surgery. J Hand Surg Br. 2004;29:4548–4560. doi: 10.1016/j.jhsb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Artzberger S., Priganc V. Manual oedema mobilization: an edema reduction technique for the orthopaedic patient. In: Skirven T.M., Osterman A.L., Fedorcczyk J.M., Amadio P.C., editors. Rehabilitation of the Hand and Upper Extremity. 6th ed. Mosby; St. Louis, MO, USA: 2011. pp. 868–881. [Google Scholar]
- 8.Casley-Smith J.R., Casley-Smith J.R. 5th ed. The Lymphoedema Association of Australia; Malvern: 1997. Modern Treatment of Lymphoedema. [Google Scholar]
- 9.Miller G.E., Seale J. Lymphatic clearance during compressive loading. Lymphology. 1981;14:161–166. [PubMed] [Google Scholar]
- 10.Artzberger S. Hand manual edema mobilization: overview of a new concept in hand edema reduction. E-Document SAJHT 1: 1, 2003. Accessed September 2, 2016.
- 11.Kurz I. 4th ed. Vol. 2. Heidelberg; Springer, Berlin, Germany: 1997. (Textbook of Dr. Vodder’s manual lymph drainage). [Google Scholar]
- 12.Palmada M., Shah S., O’Hare K. Hand Oedema: pathophysiology and treatment. Br J Ther Rehabil. 1998;5(11):556–564. [Google Scholar]
- 13.Kase K., Wallis J., Kase T. Ken Ikai Co. Ltd; Tokyo: 2003. Clinical Therapeutic Application of the Kinesio Taping Method. [Google Scholar]
- 14.Chang H.Y., Chou K.Y., Lin J.J., Wnag C.H. Immediate effect of forearm Kinesio taping on maximal grip strength and force sense in healthy collegiate athletes. Phys Ther Sport. 2010;11(4):122–127. doi: 10.1016/j.ptsp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Williams S., Whatman C., Hume P., Sheern K. Kinesio Taping in Treatment and revention of Sports Injury: A meta-analysis of the evidence for its effectiveness. Sports Med. 2012;42(2):153–164. doi: 10.2165/11594960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Thelen M.D., Dauber J.A., Stoneman P.D. The clinical efficacy of kinesio taping for shoulder pain: a randomized, double- blinded, clinical trial. J Orthop Sports Phys Ther. 2008;38(7):389–395. doi: 10.2519/jospt.2008.2791. [DOI] [PubMed] [Google Scholar]
- 17.Stupik A., Dwornik M., Bialoszweski D., Zych E. Effect of Kinesio Taping on bioelectrical activity of vastus medialis muscle: Preliminary report. Ortop Traumatol Rehabil. 2007;6:644–651. [PubMed] [Google Scholar]
- 18.Morris D., Jones D., Ryan H., Ryan C.G. The clinical effects of Kinesio® Tex taping: A systematic review. Physiother Theory Pract. 2013;29(4):259–270. doi: 10.3109/09593985.2012.731675. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDermid J.C. An introduction to evidence-based practice for hand therapists. J Hand Ther. 2004;17(2):105–117. doi: 10.1197/j.jht.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Meader N., King K., Llewellyn A. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst Rev. 2014;3:82. doi: 10.1186/2046-4053-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knygsand-Roenhoej K., Maribo T. A randomized clinical controlled study comparing the effect of modified manual edema mobilisation treatment with traditional edema technique in patients with a fracture of the distal radius. J Hand Ther. 2011;24:184–194. doi: 10.1016/j.jht.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Griffin J.W., Newsome L.S., Stralka S.Q., Wright P.E. Reduction of chronic posttraumatic hand edema: a comparison of high voltage pulsed current, intermittent pneumatic compression, and placebo treatments. Phys Ther. 1990;70:279–286. doi: 10.1093/ptj/70.5.279. [DOI] [PubMed] [Google Scholar]
- 24.Meyer-Marcotty M., Jungling O., Vaske B., Vogt P.M., Knobloch K. Standardized combined cryotherapy and compression using Cryo/Cuff after wrist arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:314–319. doi: 10.1007/s00167-010-1280-4. [DOI] [PubMed] [Google Scholar]
- 25.Roper T.A., Redford S., Tallis R.C. Intermittent compression for the treatment of the edematous hand in hemiplegic stroke: a randomized controlled trial. Age Ageing. 1999;28:9–13. doi: 10.1093/ageing/28.1.9. [DOI] [PubMed] [Google Scholar]
- 26.Flowers K.R. String wrapping versus massage for reducing digital volume. Phys Ther. 1988;68:57–59. doi: 10.1093/ptj/68.1.57. [DOI] [PubMed] [Google Scholar]
- 27.Guidice M.L. Effects of continuous passive motion and elevation on hand edema. Am J Occup Ther. 1990;44(10):914–921. doi: 10.5014/ajot.44.10.914. [DOI] [PubMed] [Google Scholar]
- 28.Faghri P.D. The effects of neuromuscular stimulation-induced muscle contraction versus elevation on hand edema in CVA patients. J Hand Ther. 1997;10:2–34. doi: 10.1016/s0894-1130(97)80008-7. [DOI] [PubMed] [Google Scholar]
- 29.Haren K., Backman C., Wilberg M. Effect of manual lymph drainage as described by Vodder on oedema of the hand after fracture of the distal radius: A prospective clinical trial. Scand J Plast Reconstr Surg Hand Surg. 2000;34:367–372. doi: 10.1080/028443100750059165. [DOI] [PubMed] [Google Scholar]
- 30.Haren K., Wilberg M. A prospective randomized controlled trial of manual lymph drainage (MLD) for the reduction of hand oedema after distal radius fracture. Br J Hand Ther. 2006;11(2):41–47. [Google Scholar]
- 31.Bell A., Muller M. Effects of kinesio tape to reduce hand edema in acute stroke. Top Stroke Rehabil. 2013;20(3):283–288. doi: 10.1310/tsr2003-283. [DOI] [PubMed] [Google Scholar]
- 32.Schulz K.F., Altman D.G., Moher D., for the CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeJong G., Horn S.D., Gassawat J.A. Towards a taxonomy of rehabilitation interventions: using an inductive approach to examine the “black box” of rehabilitation. Arch Phys Med Rehabil. 2004;85:678–686. doi: 10.1016/j.apmr.2003.06.033. [DOI] [PubMed] [Google Scholar]
- 34.DeJong G., Horn S.D., Conroy B., Nichols D., Healton E.B. Opening the black box of post-stroke rehabilitation: stroke rehabilitation patients, processes and outcomes. Arch Phys Med Rehabil. 2005;86:2. doi: 10.1016/j.apmr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Ballinger C., Ashburn A., Low J. Unpacking the black box of therapy- a pilot study to describe occupational therapy and physiotherapy interventions for people with stroke. Clin Rehabil. 1999;13:301–309. doi: 10.1191/026921599673198490. [DOI] [PubMed] [Google Scholar]
- 36.Pomeroy V.M., Niven D.S., Barrow S., Faragher E.B., Tallis R.C. Unpacking the black box of nursing and therapy practice for post-stroke shoulder pain: a precursor to evaluation. Clin Rehabil. 2001;15(1):67–83. doi: 10.1191/026921501675454995. [DOI] [PubMed] [Google Scholar]
- 37.Jackson T., Teijlingen E., Bruce J. Light retrograde massage for the treatment of post-stroke upper limb edema: clinical consensus using the Delphi technique. Br J Occup Ther. 2012;75:12. [Google Scholar]
- 38.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:7. [PMC free article] [PubMed] [Google Scholar]