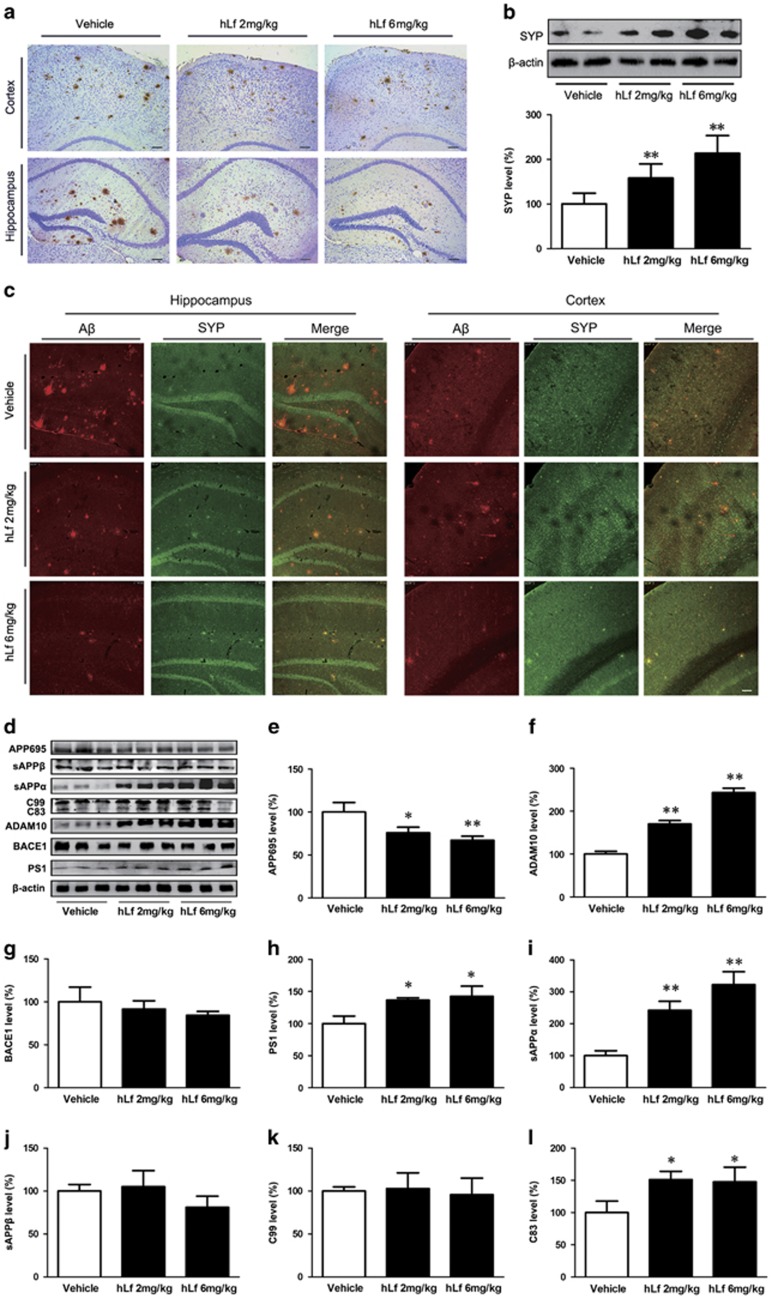
Figure 2.
Lactoferrin (Lf) treatment reduced β-amyloid (Aβ) plaque accumulation and synapse loss in amyloid precursor protein (APP)/presenilin 1 (PS1) mouse brains. (a) Immunohistochemical staining investigating the distribution of Aβ plaques in the cortex and hippocampus of the APP/PS1 mouse brains. (b) Western blot analysis demonstrated that the synaptophysin (SYP) protein levels were markedly increased in the human Lf (hLf)-treated mouse brains compared with the vehicle-treated mouse brains. β-actin was used as an internal control. (c) Immunofluorescence labeling and confocal microscopy analysis revealed the distribution and expression of anti-SYP (red) and Aβ (green) in the brain sections of the APP/PS1 mice. Scale bar=100 μm. (d) Western blot analysis showed the levels of APP cleavage enzymes and products. β-actin was used as an internal control. (e) hLf treatment resulted in significantly decreased levels of APP. (f–h) hLf treatment significantly increased the levels of ADAM10 and PS1, whereas there were no significant changes in the BACE1 levels in the brain tissues between the vehicle- and hLf-treated mice. (i–l) hLf led to an augmentation of sAPPα secretion with a concomitant increase in C83. There were no significant differences in the levels of sAPPβ and C99 in the hLf-treated mice compared with vehicle controls. All values are the means±SEM (n=8). *p<0.05, **p<0.01 compared with the vehicle group. A full color version of this figure is available at the Neuropsychopharmacology journal online.