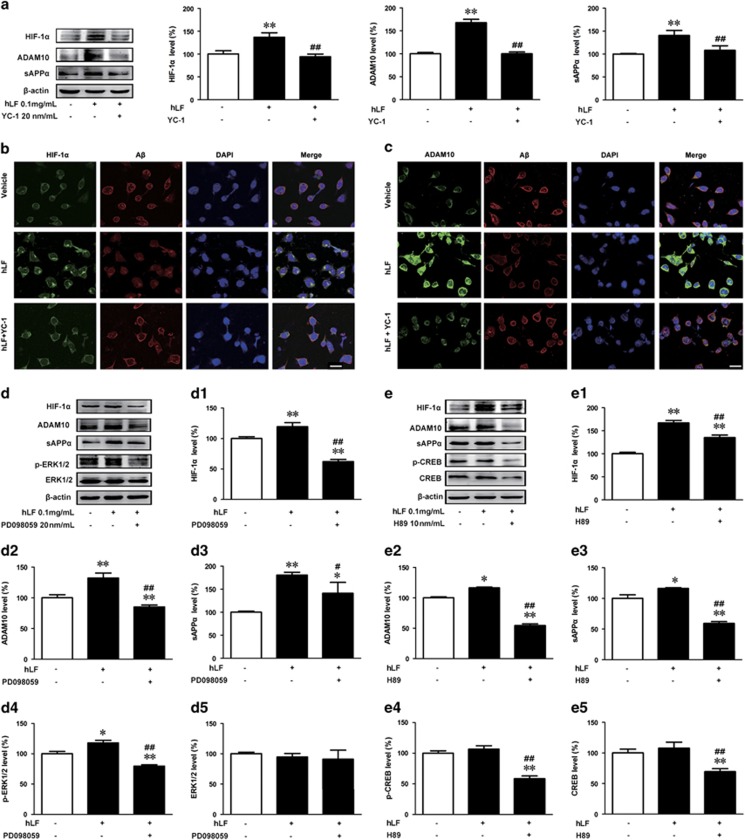
Figure 5.
ERK-CREB and hypoxia-inducible factor 1α (HIF-1α) signaling is involved in lactoferrin (Lf)-mediated promotion of amyloid precursor protein (APP) α-proteolysis in N2a cells overexpressing Swedish mutant human APP (APPsw N2a cells). (a) Cultured APPsw N2a cells were treated with human lactoferrin (hLf) in the absence or presence of a HIF-1α selective inhibitor (YC-1) for 24 h. Cell lysates were then prepared from these cells and subjected to Western blot analysis to evaluate APP processing into α-proteolysis using ADAM10, sAPP-α, and HIF-1α antibodies. β-actin was used as an internal control. (b, c) Immunofluorescence labeling using mouse anti-β-amyloid (Aβ) (red) and rabbit anti-HIF-1α/ADAM10 (green) antibodies showed that hLf likely enhanced the expression levels of HIF-1α and ADAM10 and then reduced Aβ generation in APPsw N2a cells. DAPI was used to detect the nuclei (blue). Scale bar=25 μm. APPsw N2a cells were treated for 24 h with hLf (0.1 mg/ml) in the absence or presence of the ERK1/2 inhibitor PD98059 (d) or the CREB inhibitor H89 (e). The levels of HIF-1α (D1, E1), ADAM10 (D2, E2), sAPPα (D3, E3), phosphorylated ERK1/2 (D4), and CREB (E4) and the total protein levels of ERK1/2(D5) and CREB (E5) were determined by immunoblot analysis. β-actin was used as an internal control. The data represent the means±SEM of at least three independent experiments; *p<0.05, **p<0.01 vs control group; #p<0.05, ##p<0.01 vs hLf treatment group without YC-1/PD98059/ H89 pretreatment. A full color version of this figure is available at the Neuropsychopharmacology journal online.