Abstract
Context:
Obesity is associated with subclinical white adipose tissue inflammation, as defined by the presence of crown-like structures (CLSs) consisting of dead or dying adipocytes encircled by macrophages. In humans, bariatric surgery-induced weight loss leads to a decrease in CLSs, but the effects of rapid diet-induced weight loss on CLSs and metabolism are unclear.
Objective:
To determine the effects of rapid very-low-calorie diet-induced weight loss on CLS density, systemic biomarkers of inflammation, and metabolism in obese postmenopausal women.
Design:
Prospective cohort study.
Setting:
Rockefeller University Hospital, New York, NY.
Participants:
Ten obese, postmenopausal women with a mean age of 60.6 years (standard deviation, ±3.6 years).
Main Outcome Measures:
Effects on CLS density and gene expression in abdominal subcutaneous adipose tissue, cardiometabolic risk factors, white blood count, circulating metabolites, and oxidative stress (urinary isoprostane-M) were measured.
Results:
Obese subjects lost approximately 10% body weight over a mean of 46 days. CLS density increased in subcutaneous adipose tissue without an associated increase in proinflammatory gene expression. Weight loss was accompanied by decreased fasting blood levels of high-sensitivity C-reactive protein, glucose, lactate, and kynurenine, and increased circulating levels of free fatty acids, glycerol, β-hydroxybutyrate, and 25 hydroxyvitamin D. Levels of urinary isoprostane-M declined.
Conclusion:
Rapid weight loss stimulated lipolysis and an increase in CLS density in subcutaneous adipose tissue in association with changes in levels of circulating metabolites, and improved systemic biomarkers of inflammation and insulin resistance. The observed change in levels of metabolites (i.e., lactate, β-hydroxybutyrate, 25 hydroxyvitamin D) may contribute to the anti-inflammatory effect of rapid weight loss.
Keywords: crown-like structure, weight loss, metabolism, inflammation
Rapid diet-induced weight loss increases subcutaneous adipose crown-like structure density in obese postmenopausal women.
Obesity has reached epidemic proportions, with about two-thirds of US adults classified as overweight or obese [1]. Complications of obesity include type 2 diabetes, cardiovascular disease, and increased incidence of and worsened prognosis for several cancers, including postmenopausal breast cancer [2, 3]. Inflamed adipose tissue is believed to contribute to obesity-associated complications. This is characterized by immune-cell infiltration, increased levels of proinflammatory cytokines, as well as adipocyte insulin resistance, mitochondrial dysfunction, and endoplasmic reticulum stress [4]. Crown-like structures (CLSs) are found in the white adipose tissue of both obese mice and humans. In cross-sectional studies, the presence of CLSs is associated with insulin resistance, cardiovascular disease (CVD), and worse prognosis for patients with cancer [5–7]. These inflammatory foci represent dead or dying adipocytes enveloped by macrophages. The macrophages rely on lysosomal exophagy to phagocytose the dead adipocytes and become foam cells [8, 9]. When white adipose tissue fails to expand appropriately to store excess energy, ectopic fat deposition occurs in other organs, leading to insulin resistance—a process known as lipotoxicity. The ability of the CLS macrophages to store free fatty acids may help protect against both local adipocyte dysfunction and lipotoxicity [10].
In obese rodents, prolonged caloric restriction is associated with a reduction in CLS and reduced expression of proinflammatory genes [11]. By contrast, rapid weight-loss results in increased macrophage infiltration in visceral and subcutaneous adipose tissue [12]. In humans, bariatric surgery-induced weight loss leads to a reduction in CLSs, improved systemic inflammation, and decreased insulin resistance and, possibly, cancer risk [13]. The effects of very-low-calorie diet (VLCD)-mediated rapid weight loss have also been investigated. Clément and colleagues [14] reported that VLCD-induced rapid weight loss leads to a substantial reduction in fat mass in association with improved insulin sensitivity, reduced circulating triglyceride and cholesterol levels, as well as changes in subcutaneous fat gene expression. There is considerable evidence that cross-talk between adipose tissue and distal tissues, including the liver, pancreas, and skeletal muscle, can affect health [15].
Although the effects of rapid weight loss on circulating levels of adipokines and lipids have been reported [16, 17], much less is known about the effects on systemic levels of metabolites. In this study, our primary objective was to investigate the effects of VLCD-induced weight loss on CLS density, systemic biomarkers of inflammation, and metabolism in obese postmenopausal women.
1. Materials and Methods
A. Subjects
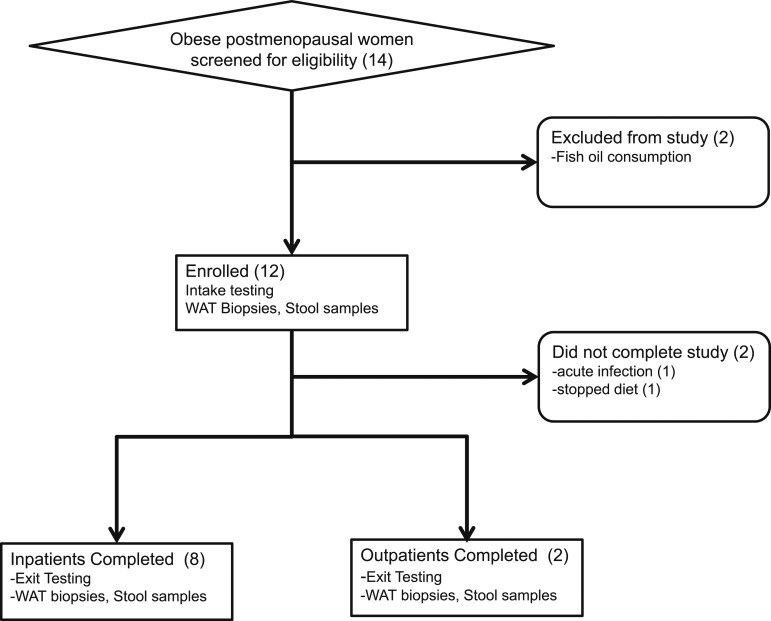
Eligible subjects were obese (body mass index, ≥35 kg/m2) postmenopausal women (defined by >2 years without menstrual periods). Participants were recruited from the community through advertising or participation in a Rockefeller Hospital registry of subjects previously screened for research studies. We excluded subjects with clinical CVD; type 2 diabetes and receiving oral hypoglycemic agents; smokers; regular users of aspirin, fish oils, or vitamin D supplements; a history of gastrointestinal surgery other than appendectomy; or breast and other cancers. Fourteen subjects were screened, and 12 of these were evaluated and enrolled. During the study, two subjects dropped out: one because of acute conjunctivitis and another for inability to adhere to the diet. Of those who completed the study, three were white, four were black, and three were Hispanic. This was a single-center study performed at The Rockefeller University Hospital between September 2012 and August 2013. Subjects underwent a complete medical examination, standard blood and urine tests, and an electrocardiogram, and all were found to be healthy. A Transparent Reporting of Evaluation with Nonrandomized Designs flowchart summarizing subject selection is presented in Figure 1.
Figure 1.
Transparent Reporting of Evaluation with Nonrandomized Designs flowchart of the study. Fourteen subjects were screened, of whom 12 were enrolled, and 10 completed the study. Two subjects completed the weight loss intervention as outpatients, and the remaining subjects completed as inpatients. WAT, white adipose tissue.
B. Sample Size
The primary end point of this study was to determine whether the intervention would significantly alter CLS density in subcutaneous adipose tissue. Sample size was primarily determined by logistics and feasibility considerations. However, with 10 subjects, assuming a coefficient of variation of 30%, a paired t test applied to this sample provided 80% power to detect an effect size as small as a 1-unit change in CLS/cm2 from before to after weight loss. This effect size is equivalent to 30% increase from baseline CLS density.
C. Trial Approval and Registration
The study was approved by the institutional review boards at Rockefeller University, Weill Cornell Medical College, and Memorial Sloan-Kettering Cancer Center, and registered under ClinicalTrials.gov identifier NCT01699906.
D. Interventions, Specimen Collection, and Analysis
The effect of the VLCD was assessed in subjects after they had lost approximately 10% of body weight. The diet of each subject before weight loss was evaluated by the Harvard Food Frequency Questionnaire, which quantifies food intake over a 3-day period [18]. On admission to the Rockefeller University Hospital, baseline study specimens were collected. Subsequently, participants underwent a 3-day dietary adjustment period, consuming 50% of their prestudy caloric intake. Of the 10 subjects who completed the VLCD-induced weight loss, two did so as outpatients with intensive monitoring and eight as inpatients. Blood samples were drawn biweekly for safety monitoring, which included measurements of electrolytes and liver function tests. The rate of weight loss did not differ significantly between those studied as outpatients or inpatients. After losing approximately 10% of their baseline body weight, all subjects consumed the VLCD for three additional days as inpatients, during which all of the baseline measurements were repeated. Bloods for end-point analyses were collected under fasting conditions. Therefore, each study subject acted as her own control.
The VLCD consisted of a commercially available diet (New Direction Program, Robard Corp., Mount Laurel, NJ) that provided approximately 800 Kcal/d with an estimated macronutrient energy distribution of 54% protein, 26% carbohydrate, 20% fat (including 4% saturated fat and 200 mg of cholesterol) and 10 g/d fiber, as previously described [19]. This commercial preparation provided a choice of shakes, soups, bars, and puddings. Subjects had four choices per day and consumed one item every four waking hours.
Fasting blood samples were analyzed in the Clinical Pathology Laboratory of Memorial Sloan-Kettering Cancer Center for electrolyte levels, liver function, renal function, lipid profile, and high-sensitivity C-reactive protein (hsCRP) level. Aliquots of serum for cytokine measurements, including interleukin (IL)-6, IL-10, and IL-17, were stored at −80°C for subsequent analysis. Enzyme-linked immunosorbent analyses of leptin, adiponectin, insulin, steroid hormone-binding globulin (SHBG), estradiol, and IL-6 were performed at the Pollak Assay Laboratory (McGill University, Quebec, Canada) [6]. Erenna immunoassay analyses (EMD Millipore, Billerica, MA) of IL-10 and IL-17 were performed by the Rockefeller Translational Technology Core Laboratory. Body composition was measured by air-displacement plethysmography using the BodPod system (COSMED, Italy). Subcutaneous adipose tissue was obtained by aspiration biopsy using a large-bore, modified liposuction needle (Anthony Products, Indianapolis, IN) under local anesthesia with lidocaine between 08:00 and 11:00. The initial adipose tissue biopsy specimen was taken in the left lower quadrant of the abdomen of each subject, whereas the final biopsy specimen was taken in the right lower quadrant abdomen. The quadrants for the two biopsies were alternated to avoid the potential of results being confounded by trauma-related to the initial biopsy. Adipose tissue was formalin fixed and paraffin embedded for immunohistochemistry or frozen in RNAlater (Ambion Inc, Austin TX) for subsequent RNA extraction and analysis.
E. Urinary F2-isoprostane-M Measurement
Analysis of urinary F2-isoprostane-M was performed on an aliquot of a 24-hour urine collection by liquid chromatography–mass spectrometry in the Vanderbilt University Eicosanoid Core Laboratory as described previously [20]. F2-isoprostane-M levels were normalized to creatinine.
F. CLS Quantification in Adipose Tissue Biopsy Specimens
CLS in abdominal subcutaneous adipose tissue biopsy specimens were quantified as described previously [6]. Biopsy samples were fixed in formalin for 24 hours and then embedded in a paraffin block. Six sections were cut with a spacing of 50 μm; the first section was stained with hematoxylin and eosin (H&E) to ensure the sample was representative of adipose tissue. The remaining five sections were stained for the macrophage marker CD68 (mouse monoclonal KP1 antibody; dilution 1:4000; Dako, Glostrup, Denmark) to identify CLS by light microscopy. The numbers of CLSs per section were recorded by the study pathologist (D.D.G.). The area of adipose tissue examined in each of the five sections was measured using Image J software (National Institutes of Health, Bethesda, MD) to calculate CLS density as CLS/cm2.
G. Adipocyte Diameter
H&E-stained sections were generated from subcutaneous adipose tissue biopsy specimens to measure adipocyte diameters, as previously described [21, 22]. The H&E-stained sections were photographed at ×20 magnification using an Olympus BX50 microscope (Orangeburg, NY) and MicroFire digital camera (Optronics Goleta, CA). Mean diameters were calculated using measurements from ≥30 individual adipocytes for each patient, using the linear dimensional tool in the Canvas 11 Software (ACD Systems International Victoria, Canada).
H. Gene Expression
Total RNA was extracted from approximately 0.5 g of frozen adipose tissue using a Qiagen RNeasy Lipid Tissue Mini Kit (Germantown, MD). RNA quality was assessed using an Agilent Bioanalyzer. Approximately, 2 to 3 μg of RNA with RNA integrity number >7 were submitted for 50-bp paired-end read RNA sequencing of polyA-enriched RNA at the New York Genome Center. Gene expression was analyzed for pathway enrichment using Gene Set Enrichment Analysis (GSEA; Broad Institute, Cambridge MA) and Ingenuity Pathway Analysis (Qiagen).
For heat map generation, genes were annotated using the biomaRt library in R package Bioconductor (https://www.bioconductor.org/). RNA-seq data were normalized by a regularized logarithmic transformation into a matrix through DESeq2 [23]. The Gene Set Variation Analysis (GSVA) algorithm [24] was applied to the matrix to quantify pathway activity for a set of 1454 curated gene sets available in the Molecular Signatures Database. Supervised heat maps were generated to visually compare pathway activities in before and after weight-loss samples, and to assess subject and pathway similarities. A linear mixed-effects model was applied to estimate averages for the pathway activity before and after weight loss and to determine the pathways that differed significantly between before and after VLCD weight loss. To achieve a more concise visual representation, these averages were displayed in a heat map (scaled column-wise) based on Euclidean distance and a complete linkage algorithm for pathway hierarchical clustering.
I. Quantitative Real-Time Polymerase Chain Reaction
Transcript expression was assessed after total RNA was isolated from adipose tissue, using the RNeasy Mini Kit (Qiagen). RNA (100 ng) was reverse transcribed using the qScript cDNA Synthesis Kit (QuantaBio Beverly, MA) and the resulting cDNA used for real-time polymerase chain reaction (PCR) amplification using Fast SYBR Green PCR master mix on a 7500 HT real-time PCR system (Applied Biosystems, Foster City, CA). IL-6 was amplified using a Qiagen QuantiTect primer assay (catalog no. QT00083720). Primers used to amplify tumor necrosis factor-α were: forward: 5′-CTG CAC TTT GGA GTG AT-3′; reverse: 5′-AGA TGA TCT GAC TGC CTG GG-3′. Primers used to amplify IL-1β were: forward: 5′-GGA CAA GCT GAG GAA GAT GC-3′; reverse: 5′-TCG TTA TCC CAT GTG TCG AA-3′. Transcript expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which was amplified using a Qiagen QuantiTect primer assay (catalog no. QT00079247). Expression was determined using the ΔΔCT analysis protocol.
J. Statistical Analyses
Two-tailed paired t tests and Wilcoxon tests were used to compare anthropometric measurements, biochemical variables, serum cytokines, and immunohistochemical results before and after VLCD-induced weight loss. P < 0.05 was considered significant. For metabolomic analyses, matched paired t tests were used to identify compounds that differed significantly between the before and after weight-loss samples, correcting for multiple measurements. GraphPad Prism and Excel were used for data visualization.
2. Results
The mean age (± standard deviation) of the 10 subjects completing the study was 60.6 ± 3.6 years. The mean time to induce ~10% weight loss was 46.2 ± 15.3 days (range, 30 to 74 days). Table 1 shows the mean macronutrient composition of the subjects’ self-selected prestudy diets and that of the VLCD consumed during weight loss. The daily protein intake of the VLCD (388 ± 45 Kcal protein per day) was similar to the subject’s prestudy diet (382 ± 28 Kcal protein per day; P = not significant), whereas fat, carbohydrate, and fiber contents were all markedly reduced.
Table 1.
Composition of the Diet Before Weight Loss and the VLCD
| preWL Diet | VLCD | |
|---|---|---|
| Calories, Kcal/d | 2324 ± 1041 | 721 ± 83 |
| % Carbohydrate | 49 ± 8 | 26 ± 1 |
| % Protein | 16 ± 3 | 54 ± 1 |
| % Fat | 36 ± 8 | 20 ± 2 |
| % Saturated fat | 11 ± 4 | 4 ± 1 |
| Fiber, g | 33 ± 18 | 10 ± 4 |
Values are presented as mean ± standard deviation.
Abbreviation: preWL, before weight loss.
A. Effects of the VLCD-Induced Weight Loss on Anthropometric and Clinical Parameters
The before and after weight loss anthropometric and clinical data are shown in Table 2. The VLCD decreased BMI by 9.6%, including a 3.8% reduction in body fat. Decreases were observed in fasting glucose (13.3%) and insulin (20.3%) levels, and the Homeostatic Model for Assessment of Insulin Resistance (30.9%), reflecting improved insulin sensitivity. High-density lipoprotein cholesterol levels decreased (16.0%), with trends toward decreased low-density lipoprotein cholesterol (8.2%) and triglyceride (19.3%) levels. Fasting leptin levels decreased (57.2%), whereas adiponectin was unaffected. Estradiol levels were unaffected by weight loss, but levels of SHBG increased (56.7%). Weight loss led to a significant increase in 25-hydroxyvitamin D (19.6%) and calcium (2.5%) levels.
Table 2.
Effects of Rapid VLCD-Induced Weight Loss
| preWL | postWL | Difference, % | P Valuea | |
|---|---|---|---|---|
| Weight, kg | 101.3 ± 9.7 | 90.9 ± 8.2 | −10.3 | <0.01 |
| BMI, kg/m2 | 38.8 ± 3.4 | 35.1 ± 3.0 | −9.6 | 0.01 |
| Waist, cm | 115.8 ± 6.3 | 107.5 ± 7.9 | −7.2 | 0.01 |
| % Body fat composition | 51.4 ± 6.1 | 47.6 ± 5.5 | −7.4 | <0.01 |
| Metabolic parameters | ||||
| Glucose, mg/dL | 103.4 ± 17.6 | 89.6 ± 14.9 | −13.3 | 0.01 |
| Insulin, mU/mL | 12.3 ± 1.8 | 9.8 ± 2.1 | −20.3 | 0.07 |
| HOMA-IR | 3.30 ± 0.59 | 2.28 ± 0.60 | −30.9 | 0.04 |
| HDL, mg/dL | 54.3 ± 10.3 | 45.6 ± 6.2 | −16.0 | 0.02 |
| LDL, mg/dL | 131.2 ± 24.8 | 120.5 ± 27.0 | −8.2 | 0.08 |
| TG, mg/dL | 96.9 ± 28.8 | 78.2 ± 25.0 | −19.3 | 0.08 |
| Leptin, ng/mL | 61.0 ± 21.0 | 26.1 ± 12.2 | −57.2 | <0.001 |
| Adiponectin, μg/mL | 9.8 ± 5.8 | 10.2 ± 5.5 | 4.1 | 0.20 |
| Estradiol, pg/mL | 36.2 ± 14.3 | 33.4 ± 11.6 | −7.7 | 0.38 |
| SHBG, nmol/L | 60.3 ± 26.3 | 94.5 ± 33.7 | 56.7 | <0.001 |
| Calcium, mg/dL | 9.06 ± 0.27 | 9.29 ± 0.31 | 2.5 | 0.01 |
| 25-OH vitamin D, ng/mL | 22.5 ± 10.3 | 26.9 ± 10.3 | 19.6 | 0.04 |
| Inflammatory parameters | ||||
| WBC, 103/uL | 5.96 ± 2.00 | 4.92 ± 1.56 | −17.4 | 0.01 |
| Neutrophils, 103/μL | 3.22 ± 0.60 | 2.31 ± 0.54 | −28.3 | 0.01 |
| hsCRP, mg/dL | 0.68 ± 0.42 | 0.44 ± 0.24 | −35.3 | 0.01 |
| IL-6, pg/mL | 3.1 ± 1.7 | 2.6 ± 1.1 | −16.1 | 0.07 |
| IL-10, pg/mL | 1.49 ± 0.99 | 1.31 ± 0.76 | −12.1 | 0.50 |
| IL-17, pg/mL | 0.22 ± 0.10 | 0.24 ± 0.16 | 9.1 | 0.38 |
| MCP-1, pg/mL | 312 ± 130 | 292 ± 115 | −6.4 | 0.32 |
| F2-IsoP-M, ng/mg Cr | 1.51 ± 0.57 | 0.89 ± 0.29 | −41.1 | 0.01 |
Values are presented as mean ± standard deviation.
Abbreviations: 25-OH vitamin D, 25 hydroxyvitamin D; BMI, body mass index; F2-IsoP-M, F2-isoprostane-M; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model for Assessment of Insulin Resistance; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein 1; TG, triglyceride; WBC, white blood cell count.
Significance was determined by Wilcoxon signed-rank test.
B. Effects of the VLCD-Induced Weight Loss on Systemic Markers of Inflammation and Oxidant Stress
VLCD-induced weight loss was associated with decreased levels of hsCRP (35.3%) and total white blood cell count (17.4%), largely due to fewer circulating neutrophils (28.3%) (Table 2). Levels of urinary F2 isoprostane-M, a biomarker of oxidant stress, decreased by 41.1% following VLCD-induced weight loss.
C. Effects of the VLCD-Induced Weight Loss on Subcutaneous Adipose Tissue
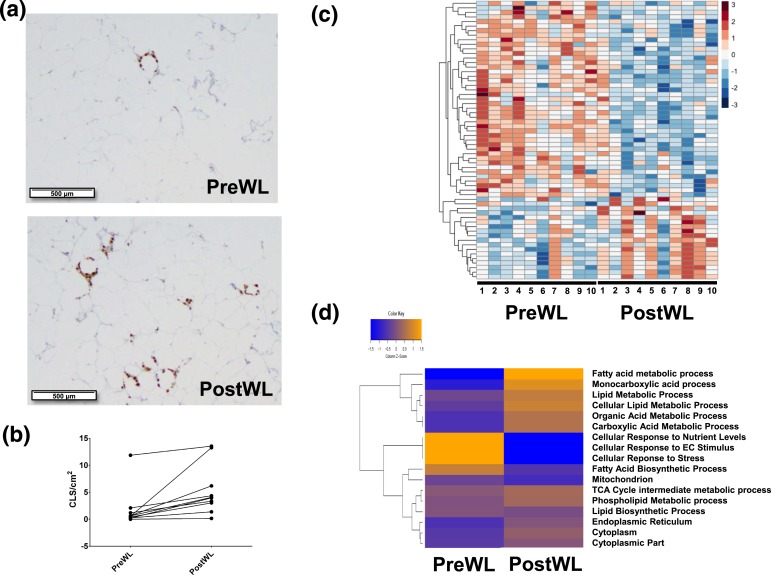
Mean adipocyte diameter was 111.1 ± 13.0 μm at baseline and 111.7 ± 11.3 μm after weight loss. We also assessed the density of CLSs in abdominal subcutaneous adipose tissue at baseline and after weight loss [Fig. 2(a)]. CLSs were detected in all 10 subjects at baseline. Interestingly, the density of CLSs increased from a mean of 1.78 ± 1.04 to 5.33 ±1.32 CLS/cm2 after weight loss [P = 0.01; Fig. 2(b)].
Figure 2.
Effects of VLCD-induced weight loss on subcutaneous adipose tissue. Biopsy specimens were obtained before and after weight loss and subjected to CD68 immunohistochemistry and RNA sequencing. (a) Subcutaneous adipose tissue images from the subject with the largest increase in CLSs after weight loss are shown. (Top panel) preWL. (Bottom panel) postWL. (b) Line plot of CLS density (CLS/cm2) shown from preWL to postWL in each individual subject (P = 0.01). (c) Heat map for 61 genes whose expression changed significantly from preWL to postWL. Subjects (columns) are arranged from preWL to postWL. Genes (rows) are clustered hierarchically in a supervised manner. (d) Heat map of expression of 17 lipid metabolism gene pathways altered significantly between preWL and postWL. postWL, after weight loss; preWL, before weight loss.
To further explore the effects of VLCD-induced weight loss on the biology of subcutaneous adipose tissue, RNA sequencing was carried out on adipose tissue obtained before and after weight loss. A total of 61 genes were differentially expressed at absolute log fold-change >2 and P < 0.001 (Supplemental Table 1 (58KB, docx) ). Of the differentially expressed genes, about two-thirds decreased and one-third increased after weight loss [Fig. 2(c)]. GSEA identified overrepresented metabolic pathways relating to triacylglycerol and ketone metabolism, and cellular respiration. As shown in Figure 2(d), analysis by GSVA showed that VLCD-induced weight loss was associated with increased expression of genes involved in metabolism of monocarboxylic acids, fatty acids, the tricarboxylic acid cycle, phospholipids, lipids, organic acid, and carboxylic acids; and decreased expression of genes involved in fatty acid biosynthesis, cellular responses to nutrient levels, extracellular signaling, and to stress. Additionally, pathway analyses, including GSEA, Ingenuity Pathway Analysis, and GSVA showed that VLCD-induced weight loss did not affect the expression of genes related to inflammation. To confirm this finding, quantitative real-time PCR was carried out. Levels of tumor necrosis factor-α, IL-1β, and IL-6 mRNAs were unaffected by VLCD-induced weight loss (data not shown).
D. Effects of VLCD-Induced Weight Loss on the Plasma Metabolome
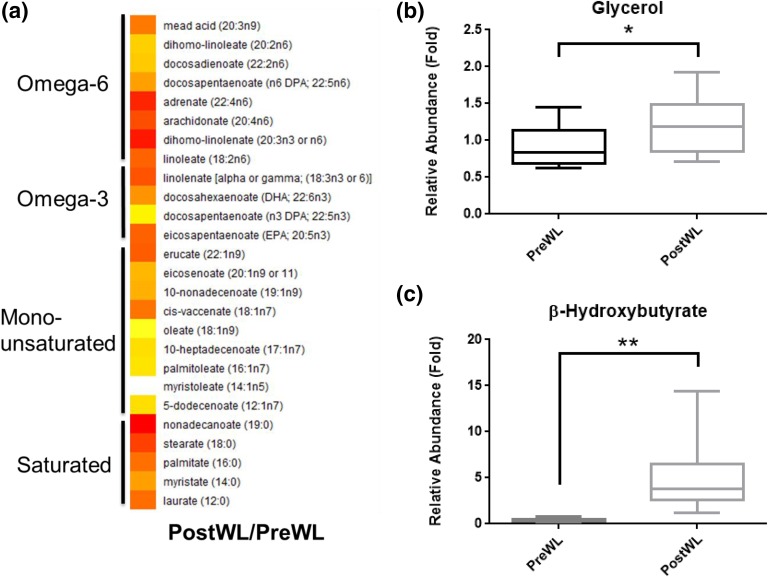
To determine the effects of VLCD-induced weight loss on circulating metabolites, plasma collected under fasting conditions was subjected to targeted metabolomic analysis. A total of 336 metabolites were detected. Of these, 131 metabolites changed significantly, with 67 increasing (Supplemental Table 2 (58KB, docx) ) and 64 decreasing (Supplemental Table 3 (58KB, docx) ). The decrease in plasma levels of creatine and 3-methylhistidine after VLCD-induced weight loss is consistent with this diet being protein sparing (Table 3). The observed increase in both circulating free fatty acid and glycerol levels after treatment with the VLCD suggests enhanced lipolysis [Fig. 3(a) and 3(b)]. Treatment with the VLCD also led to elevated ketone levels, including a 16-fold increase in β-hydroxybutyrate and a 7.6-fold increase in acetoacetate (Table 3). Levels of lactate, tryptophan, and the tryptophan metabolite kynurenine all decreased after VLCD-induced weight loss. By contrast, the metabolites p-cresol sulfate and glycolithocholate sulfate increased after VLCD-induced weight loss, consistent with altered intestinal bacterial metabolism (Table 3).
Table 3.
VLCD-Induced Weight Loss Was Associated With Changes in Levels of Plasma Metabolites
| Increased Plasma Metabolite | Mean Pairs Ratio PostWL/PreWL | P Value | Decreased Plasma Metabolite | Mean Pairs Ratio PostWL/PreWL | I Value |
|---|---|---|---|---|---|
| β-Hydroxybutyrate | 15.98 | <0.001 | 3-Methylhistidine | 0.63 | 0.01 |
| p-Cresol sulfate | 10.05 | 0.03 | Creatine | 0.69 | <0.01 |
| Glycolithocholate sulfate | 8.76 | 0.03 | Kynurenine | 0.74 | <0.001 |
| Acetoacetate | 7.64 | <0.001 | Lactate | 0.74 | <0.01 |
| Tryptophan | 0.78 | <0.001 |
Values are presented as mean pairs ratio of postWL vs preWL levels.
Abbreviations: PostWL, after weight loss; PreWL, before weight loss.
Figure 3.
Effects of VLCD-induced weight loss on levels of free fatty acids and glycerol in plasma. (a) Heat map comparing the ratios of plasma free fatty acid levels between postWL and preWL samples. White indicates unity, with yellow to red indicating increased levels. All free fatty acid species shown were significantly increased (P < 0.05). (b) Box plot of preWL and postWL abundance of plasma glycerol relative to total ion counts, and scaled by setting the preWL loss median to one (*P = 0.01). postWL, after weight loss; preWL, before weight loss.
3. Discussion
In this study, we determined the effects of VLCD-induced rapid weight loss on both inflammation and metabolism in obese postmenopausal women. Consistent with the known anti-inflammatory effects of weight loss [25], VLCD-induced rapid weight loss resulted in decreased levels of hsCRP, reduced white blood cell count, and a near-significant reduction in circulating IL-6 levels [26]. Obesity has been associated with oxidant stress, which can also contribute to inflammation [27]. We observed decreased levels of urinary F2-isoprostane-M after weight loss, suggesting decreased oxidative stress [28].
The density of CLS, a histological marker of adipose inflammation, is increased in both visceral and subcutaneous adipose tissue from obese vs lean subjects and correlates with elevated levels of circulating proinflammatory mediators [29]. To complement our analysis of systemic markers of inflammation, we explored the effects of rapid VLCD-induced weight loss on CLS density in subcutaneous adipose tissue. Interestingly, VLCD-induced weight loss was associated with a significant increase in CLS density in the absence of a change in adipocyte size or expression of proinflammatory genes. Weight loss after bariatric surgery has been associated with a reduction in CLS [13]. By contrast, in obese mice, Kosteli et al. [12] showed that rapid weight loss stimulated lipolysis, resulting in the accumulation of macrophages in adipose tissue [12]. Elevated levels of free fatty acids, a consequence of increased lipolysis, were found to function as chemoattractants, leading to increased macrophage content. We also observed a significant increase in lipolysis after VLCD-induced weight loss. Taken together, we speculate that VLCD-mediated rapid weight loss stimulated lipolysis, contributing to the observed increase in CLS density.
The increase in CLS density in response to VLCD-induced weight loss has potentially significant implications. These CLS macrophages can store excess free fatty acids [29, 30], thereby protecting against lipotoxicity and contributing to the observed improvement in insulin sensitivity and decreased systemic inflammation following rapid weight loss. There are other possible explanations for how rapid VLCD-induced weight loss could lead to both an increase in CLS density in abdominal subcutaneous fat and a decrease in systemic inflammatory biomarkers. One possibility is that rapid weight loss has different effects on visceral vs subcutaneous fat. Perhaps a decrease in CLS density occurred in visceral fat, contributing to reduced systemic inflammation. Another possibility is that the overall decrease in fat mass mediated by the VLCD led to a decrease in systemic inflammation. A limitation of our study is that the effects of rapid weight loss were only measured at a single time point when fat remodeling was occurring. It is possible that a decrease in CLS density would be observed later, once a new steady state is achieved.
Although weight loss was associated with a small increase in CLS density, we did not detect a comparable increase in the expression of proinflammatory genes. There are multiple potential explanations for this finding. Lactate has been observed to stimulate M1 polarization of macrophages [31]. Plasma lactate levels were reduced after rapid weight loss, presumably reflecting improved adipose tissue oxygenation [32]. Because lactate has been reported to stimulate M1 polarization of macrophages, a fall in adipose tissue lactate levels in concert with reduced circulating levels could prevent an increase in proinflammatory gene expression. We also observed a 16-fold increase in β-hydroxybutyrate after weight loss. Youm et al. [33] found that β-hydroxybutyrate blocked inflammation mediated by the NLRP3 inflammasome. It is possible, therefore, that increased β-hydroxybutyrate levels contributed to the lack of a local inflammatory phenotype in subcutaneous adipose tissue. Consistent with findings in other weight-loss studies [34], we observed an increase in circulating 25 hydroxyvitamin D levels after VLCD-induced weight loss. Because vitamin D possesses anti-inflammatory properties [35], the increase in 25 hydroxyvitamin D levels might also help to explain the lack of increased proinflammatory gene expression after VLCD-induced weight loss.
We also determined the effects of VLCD-induced weight loss on a variety of metabolic end points. Insulin sensitivity improved, as shown by a reduced Homeostatic Model for Assessment of Insulin Resistance index. The VLCD diet was associated with reduced levels of both high-density lipoprotein cholesterol and triglycerides, which, together with a trend toward decreased low-density lipoprotein cholesterol levels, probably makes the changes neutral with respect to risk of CVD [36]. Estradiol levels were low, reflecting the postmenopausal state, and did not change after VLCD-induced weight loss. However, SHBG levels increased after treatment with the VLCD, which would be predicted to decrease free estradiol. In all likelihood, the increase in SHBG levels after VLCD-induced weight loss reflects improved insulin sensitivity [37]. Consistent with the observed decrease in fat mass, levels of leptin, a proinflammatory adipokine, declined by >50% after VLCD-induced weight loss. This finding is consistent with the recent report of Magkos et al. [38] who showed that 5.1% to 16.4% weight loss led to a progressive decline in circulating leptin levels. Increased circulating levels of estradiol, insulin resistance, and elevated levels of leptin have been suggested to contribute to the increased risk of breast cancer in obese postmenopausal women [39]. The changes in free estrogens, insulin resistance, and leptin levels after rapid VLCD-induced weight loss collectively may lower the risk of breast cancer in this population [40]. Obesity is also associated with numerous immunosuppressive effects [41]. Kynurenine, an endogenous ligand of the aryl hydrocarbon receptor, has well-documented immunomodulatory effects, including stimulating naïve T-cell polarization to the regulatory T-cell phenotype [42]. VLCD-induced weight loss was accompanied by reductions in circulating levels of tryptophan and kynurenine, a tryptophan metabolite. Based on these metabolic changes, future studies are warranted to evaluate the effects of rapid weight loss on immune function.
In summary, we showed in obese postmenopausal women that rapid diet-induced weight loss was associated with increased CLS density with concomitant improved insulin sensitivity and decreased circulating biomarkers of inflammation. The increase in CLS density is likely to reflect adipose remodeling. In cross-sectional studies, we and others have shown that obesity-associated CLSs are associated with diabetes, CVD, and worse cancer prognosis. The current study illustrates the dynamic nature of CLS and highlights the potential importance of the rate of weight loss on adipose tissue biology.
Acknowledgments
We thank the clinical research staff and research volunteers who made this work possible. We acknowledge Juana Gonzalez and the Translational Technology Core Laboratory at Rockefeller University Hospital for IL-10 and IL-17 measurements.
Current affiliation: J.O. Alemán’s current affiliation is Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, NYU Langone Medical Center, 522 First Ave, New York, NY 10016. C.A. Huddis’ current affiliation is American Society of Clinical Oncology, 2318 Mill Rd, Suite 800, Alexandria, VA 22314.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences Grant UL1 TR000043, National Institutes of Health Clinical and Translational Science Award program to Rockefeller University, the Rockefeller University Center for Basic and Translational Research on Disorders of the Digestive System, from the Leona M. and Harry B. Helmsley Charitable Trust, The Rockefeller University Sackler Center for Biomedicine and Nutrition from La Fondation Sackler, the Breast Cancer Research Foundation, the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick), and the Memorial Sloan-Kettering Cancer Center Core Grant (P30 CA008748).
Author contributions: J.O.A., N.M.I., C.A.H., J.L.B., P.R.H., and A.J.D. designed the clinical study. J.O.A. and J.M.W. collected clinical study end points. J.O.A., N.M.I., J.M.W., G.L.M., J.C.R., Y.L., D.D.G., X.K.Z., and M.N.P. analyzed the data. J.O.A., J.L.B., P.R.H., and A.J.D. wrote the manuscript. J.L.B. and A.J.D. edited the manuscript.
Clinical trial registry: ClinicalTrials.gov no. NCT01699906 (registered 12 September 2012).
Disclosure summary: The authors have nothing to disclose.
Footnotes
- CLS
- crown-like structure
- CVD
- cardiovascular disease
- GSEA
- Gene Set Enrichment Analysis
- GSVA
- Gene Set Variation Analysis
- H&E
- hematoxylin and eosin
- hsCRP
- high-sensitivity C-reactive protein
- PCR
- polymerase chain reaction
- SHBG
- steroid hormone-binding globulin
- VLCD
- very-low-calorie diet.
References and Notes
- 1.Institute of Medicine Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. Washington, DC: National Academies Press; 2012. [Google Scholar]
- 2.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–2578. [DOI] [PubMed] [Google Scholar]
- 3.Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146(2):357–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21(12):1443–1455. [DOI] [PubMed] [Google Scholar]
- 5.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28(9):1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, Morrow M, Wang H, Pollak M, Jones LW, Hudis CA, Dannenberg AJ. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22(9):2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyengar NM, Ghossein RA, Morris LG, Zhou XK, Kochhar A, Morris PG, Pfister DG, Patel SG, Boyle JO, Hudis CA, Dannenberg AJ. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer. 2016;122(24):3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18(6):816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ, Maxfield FR. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res. 2016;57(6):980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, Giri D, Hudis CA, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila). 2013;6(4):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120(10):3466–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. [DOI] [PubMed] [Google Scholar]
- 14.Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18(14):1657–1669. [DOI] [PubMed] [Google Scholar]
- 15.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1-2):20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capel F, Klimcáková E, Viguerie N, Roussel B, Vítková M, Kováciková M, Polák J, Kovácová Z, Galitzky J, Maoret JJ, Hanácek J, Pers TH, Bouloumié A, Stich V, Langin D. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes. 2009;58(7):1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kováčiková M, Sengenes C, Kováčová Z, Šiklová-Vítková M, Klimčáková E, Polák J, Rossmeislová L, Bajzová M, Hejnová J, Hněvkovská Z, Bouloumié A, Langin D, Štich V. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int J Obes. 2011;35(1):91–98. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 19.Pendyala S, Neff LM, Suárez-Fariñas M, Holt PR. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, Wen W, Rothman N, Cai H, Li H, Xiang Y, Chow WH, Zheng W. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women’s Health Study. J Clin Oncol. 2009;27(15):2482–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila). 2011;4(7):1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, Falcone DJ, Krasne MD, Vahdat LT, Subbaramaiah K, Morrow M, Hudis CA, Dannenberg AJ. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res (Phila). 2015;8(5):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799–1804. [DOI] [PubMed] [Google Scholar]
- 26.Chen SB, Lee YC, Ser KH, Chen JC, Chen SC, Hsieh HF, Lee WJ. Serum C-reactive protein and white blood cell count in morbidly obese surgical patients. Obes Surg. 2009;19(4):461–466. [DOI] [PubMed] [Google Scholar]
- 27.Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ; Framingham Study . Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. [DOI] [PubMed] [Google Scholar]
- 28.Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB Jr, Wagenknecht LE. Urinary F2-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity (Silver Spring). 2012;20(9):1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, Chen A, Blüher M, Shai I, Rudich A. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. 2013;98(3):1173–1181. [DOI] [PubMed] [Google Scholar]
- 31.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rock CL, Emond JA, Flatt SW, Heath DD, Karanja N, Pakiz B, Sherwood NE, Thomson CA. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring). 2012;20(11):2296–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004; 80(6, Suppl)1717S–1720S. [DOI] [PubMed] [Google Scholar]
- 36.Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8(6):737–741. [DOI] [PubMed] [Google Scholar]
- 37.Winters SJ, Gogineni J, Karegar M, Scoggins C, Wunderlich CA, Baumgartner R, Ghooray DT. Sex hormone-binding globulin gene expression and insulin resistance. J Clin Endocrinol Metab. 2014;99(12):E2780–E2788. [DOI] [PubMed] [Google Scholar]
- 38.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las Fuentes L, He S, Okunade AL, Patterson BW, Klein S. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016;23(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. [DOI] [PubMed] [Google Scholar]
- 40.Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, Mason C, Imayama I, Kong A, Xiao L, Bain CE, Blackburn GL, Stanczyk FZ, McTiernan A. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30(19):2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James BR, Tomanek-Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol. 2012;189(3):1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. [DOI] [PubMed] [Google Scholar]