Abstract
Nicotinamide adenine dinucleotide (NAD+) is an established cofactor for enzymes serving cellular metabolic reactions. More recent research identified NAD+ as a signaling molecule and substrate for sirtuins and poly-adenosine 5′-diphosphate polymerases; enzymes that regulate protein deacetylation and DNA repair, and translate changes in energy status into metabolic adaptations. Deranged NAD+ homeostasis and concurrent alterations in mitochondrial function are intrinsic in metabolic disorders, such as type 2 diabetes, nonalcoholic fatty liver, and age-related diseases. Contemporary NAD+ precursors show promise as nutraceuticals to restore target tissue NAD+ and have demonstrated the ability to improve mitochondrial function and sirtuin-dependent signaling. This review discusses the accumulating evidence for targeting NAD+ metabolism in metabolic disease, maps the different strategies for NAD+ boosting, and addresses the challenges and open questions in the field. The health potential of targeting NAD+ homeostasis will inform clinical study design to identify nutraceutical approaches for combating metabolic disease and the unwanted effects of aging.
Keywords: nicotinamide riboside, nicotinamide mononucleotide, mitochondria, diabetes, nonalcoholic fatty liver disease, aging
This review covers recent developments in NAD+ biology and the advent of nutraceutical molecules that inform on the pathophysiology of metabolic disorders, potentially illuminating areas of therapeutic benefit.
Nicotinamide adenine dinucleotide (NAD+) was discovered more than 100 years ago by Sir Arthur Harden as a low-molecular-weight substance present in boiled yeast extracts [1]. In the late 1920s, Joseph Goldberger fed Brewer’s yeast to dogs with pellagra, a devastating disease characterized by dermatitis, diarrhea, dementia, and death, and their health improved. At that time, pellagra was endemic in parts of the United States, and so the Red Cross supplemented Brewer’s yeast to its food rations in pellagra-endemic areas; within weeks the disease burden dissipated [2, 3]. The health significance of NAD+ was established in 1937, when Conrad Elvehjem and his colleagues made the major discovery that the factor that prevented and cured pellagra was the NAD+ precursor, nicotinic acid [4, 5].
NAD+ plays a central role in cellular respiration, the cascade of reactions that generate adenosine triphosphate (ATP) from nutrient breakdown, by acting as a coenzyme for oxidoreductases and dehydrogenases [6–9]. As coenzymes, NAD+ and its phosphorylated and reduced forms, including NADP+, NADH, and NADPH, are critical for the activities of cellular metabolism and energy production [1, 10, 11]. NAD+ most commonly functions in energy-generating catabolic reactions (such as glycolysis, fatty oxidation, and citric acid cycle), where it is reduced to NADH, which is then shuttled into the mitochondria to generate ATP. This generates an NAD+/NADH ratio, which is useful to assess the health and energy charge of the cell. The phosphorylated form, NADP(H)+, participates in anabolic reactions, such as fatty acid and cholesterol synthesis [8, 9, 12].
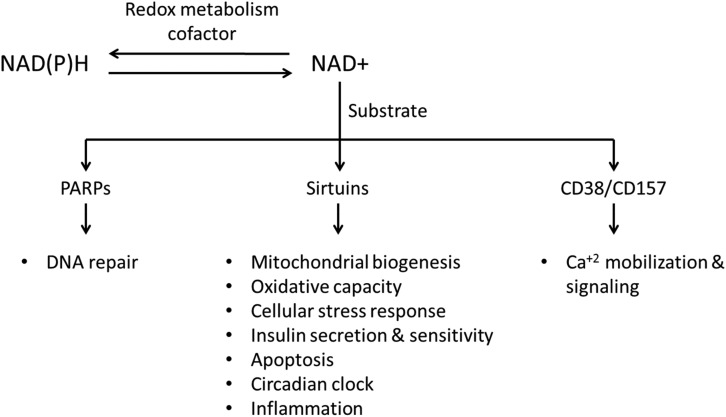
More recently and as importantly, NAD+ has been studied as a rate-limiting substrate for three classes of enzymatic reactions involved in posttranslational modification (Fig. 1), all of which exhibit breaking of the glycoside bond between nicotinamide and the adenosine 5′-diphosphate (ADP)-ribose moiety, and the latter is then transferred onto an acceptor molecule [6–9, 11]. The first class includes mono- and poly-ADP ribose transferases, among which the poly-ADP ribose polymerases (PARPs) are the most studied and are classically described as DNA repair proteins [13, 14]. The second class is the cyclic ADP ribose synthases (CD38 and CD157), which are membrane-bound ectoenzymes that produce and hydrolyze the Ca2+-mobilizing second messenger cyclic ADP-ribose from NAD+ and are therefore key in calcium homeostasis and signaling [15]. The third and most important class in terms of cellular energy metabolism consists of the sirtuins, named for their similarity to the yeast Sir2 gene-silencing protein. Seven sirtuins exist in mammals (SIRT1 through SIRT7), with diverse enzymatic activities, expression patterns, cellular localizations, and biological functions [16]. Sirtuins have a host of metabolic targets, resulting in profound effects on various cellular processes, such as mitochondrial biogenesis, cellular stress response, lipid metabolism, insulin secretion and sensitivity, apoptosis, circadian clock dynamics, inflammation, and aging [17]. Through these targets, sirtuins translate changes in feeding status, DNA damage, and oxidative stress into metabolic adaptations [18–20]. SIRT1, the most-studied sirtuin, targets multiple transcriptional coactivators, such as the peroxisome proliferator-activated receptor γcoactivator-1α (PGC-1α) and transcription factors, such as the forkhead box protein O1. PGC-1α is a central regulator of energy metabolism and mitochondrial biogenesis [21–24], whereas forkhead box protein O1 regulates mitochondrial fatty acid metabolism and protects against oxidative stress [25–27]. As nutrients influence the NAD+/NADH pool, these NAD+-dependent signaling reactions are recognized as the sensors of metabolism owing to their decisive regulatory roles in cellular metabolism [17]. Appropriate regulation of these NAD+-dependent processes relies on the cellular ability to conserve their NAD+ content. Therefore, inadequate NAD+ homeostasis can be pathologic, linked to impaired cell signaling and mitochondrial function [19, 28, 29].
Figure 1.
NAD+ as a redox cofactor and a consumed substrate.
The dependency of sirtuins on NAD+ [30], and the finding that yeast Sir2 protein is required for the lifespan extension mediated by caloric restriction (CR) [31], led to a renascent interest in NAD+ metabolism research, centered on modifying NAD+ availability to support sirtuin-mediated cellular metabolism to mimic CR. This interest was enhanced by the discovery of contemporary NAD+ precursors that can circumvent issues with existing molecules, which can also increase NAD+ in vivo and human tissues [32–34]. As we review here, these key findings underline the prospect of targeting NAD+ biosynthetic pathways to increase mitochondrial function and sirtuin activity in the combat against metabolic disease. We also highlight the challenges and the knowledge gaps that require investigating before these compounds can find their way to the clinics.
1. NAD+ Biosynthesis and Metabolism
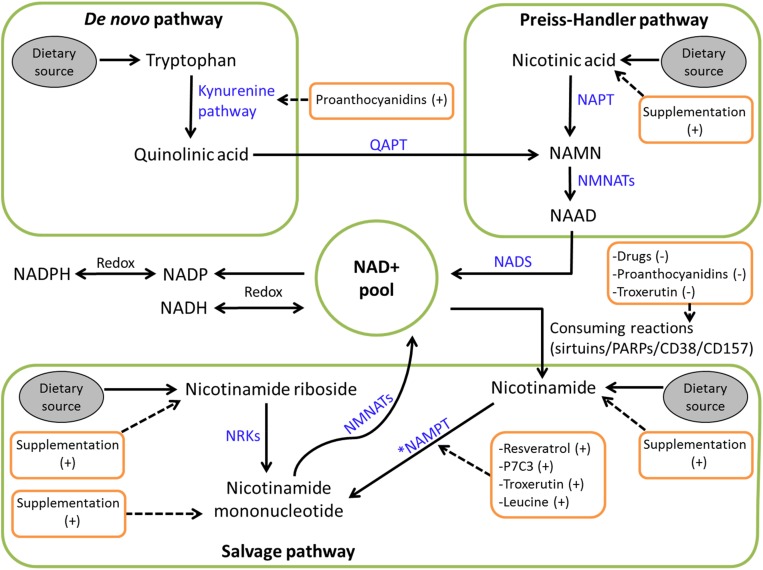
In humans, NAD+ can be synthesized via the de novo/kynurenine pathway from the amino acid tryptophan [35, 36]. However, tryptophan is a poor NAD+ precursor in vivo [37]. Most organisms have alternative NAD+ synthesis pathways (Fig. 2) from the dietary vitamin B3 precursors nicotinic acid (NA), nicotinamide (Nam), and nicotinamide riboside (NR), or from a salvage pathway where the Nam molecule split from NAD+-consuming reactions is recycled into nicotinamide mononucleotide (NMN) via the rate-limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT), and NAD+ is regenerated [9, 11, 35, 38–41]. In addition, a more recently described salvage pathway recycles NR to NMN via the nicotinamide riboside kinases (NRKs) [32]. In humans, these different routes to NAD+ synthesis converge at the NAD+ and nicotinic acid adenine dinucleotide formation step catalyzed by the nicotinamide mononucleotide adenylyltransferases. Nicotinic acid adenine dinucleotide is then amidated to form NAD+.
Figure 2.
Schematic overview of human NAD+ biosynthesis. NAAD, nicotinic acid adenine dinucleotide; NADS, NAD+ synthase; NAPT, nicotinic acid phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenylyltransferase; QAPT, quinolinic acid phosphoribosyltransferase. *NAMPT is the rate limiting step in NAD+ biosynthesis.
Nicotinic acid riboside is an NAD+ biosynthesis intermediate that can be converted in yeast and human cells by NRKs into nicotinic acid mononucleotide and then to NAD+ [42]. It is the least-studied NAD+ precursor and is therefore beyond the scope of this review.
The energy sensor adenosine monophosphate-activated protein kinase (AMPK), which adapts cells to low-energy states in the support of ATP production [43, 44], activates NAMPT, increases NAD+ recycling, and enhances SIRT1 activity [45, 46].
In mammals, the entire NAD+ pool is used and replenished several times a day, balanced by the distinct NAD+ biosynthetic pathways [47]. Owing to its constant utilization, the half-life of NAD+ in mammals is short (up to 10 hours) [36, 48–51], with intracellular levels believed to be 0.4 to 0.7 mM [41]; however, the accuracy of this level depends on the cell type and physiologic state being assessed. It is clear that NAD+ concentrations differ substantially between cellular compartments, with mitochondrial NAD+ concentration being the highest and representing 70% to 75% of cellular NAD+ (10- to 100-fold higher than those in the cytosol) [52, 53]. The NAD+/NADH levels vary to adjust cellular and tissue physiology in response to changes in nutrient availability and energy demand. For instance, NAD+ levels drop in response to high-fat diet (HFD) in mice [33, 54] and with aging, contributing to age-related disorders, such as diabetes, cardiovascular disease, cancer, and neurodegenerative disease [55–58]. Conversely, the renowned health adaptive beneficial effects of CR and exercise have been linked to NAMPT activation and the subsequent rise in NAD+, sirtuins, and mitochondrial activity [46, 59–61].
2. Therapeutic NAD+ Boosting
The recommended daily allowance (RDA) of niacin, a collective term for NA and Nam, is around 15 mg/d and can be met through the consumption of meat, fish, and dairy products [12, 62]. More recently, NR was also detected in milk and yeast [32, 63].
A plethora of evidence suggests that higher rates of NAD+ synthesis can positively affect pathways that require NAD+ as a cosubstrate. The NAD+ pools can be elevated via provision of precursors [33, 54, 64, 65], NAD+ biosynthesis augmentation [45, 46], and inhibition of NAD+ consumers [57, 66–68].
3. NAD+ Precursor Supplementation
The most tractable approach to increase NAD+ would be via the supplementation of the different precursors, all of which increase NAD+ levels in human and animal tissues. This approach is the focus of this review because NAD+ precursors are naturally occurring in food and are readily available in isolated forms, allowing nutritional approaches to be applied to modulate NAD+ metabolism in vivo.
A. Niacin
NA has been used for >50 years in the treatment of hyperlipidemia [69, 70]. Dietary niacin is not associated with side effects because the tolerable upper intake level is not exceeded [62], whereas pharmacologic NA dosing is commonly associated with undesirable effects, thereby decreasing treatment adherence. NA is a ligand for the G-protein–coupled receptor GPR109A and is coexpressed on the epidermal Langerhans cells mediating prostaglandin formation, which induces troublesome flushing and other vasodilatory effects, such as itching, hypotension, and headaches [12, 71–73]. To overcome these problems, the selective antagonist of prostaglandin D2 receptors, laropiprant, was introduced into clinical practice in combination with extended-release NA (extended-release NA-laropiprant) [74]. Extended-release NA-laropiprant failed to prove advantageous in clinical trials; safety concerns arose, and the agent was therefore withdrawn from all markets [75]. A long-acting NA analog, acipimox, is undergoing clinical trials [76–79]. However, acipimox remains a GPR109A receptor ligand [80], thus retaining the potential for undesirable side effects that will limit its clinical utility.
Although Nam is the predominant endogenous precursor of the NAD+ salvage pathway, early reports suggested that it may not be as effective as other biosynthesis precursors in increasing NAD+ levels [41]; however, this likely reflects the relatively small dose of Nam used. Additionally, Nam effects likely depend on cell/tissue type and the pathophysiologic state. For instance, in a nonstressed state, Nam is inferior to NA as an NAD+ precursor in the liver [81], whereas under HFD-induced metabolic challenge, Nam is a more powerful NAD+ precursor and SIRT1 activator than NA [82]. Nam has been used for many years for a variety of therapeutic applications (such as diabetes mellitus) at doses up to 3 g/d, with minimal side effects [83]. Unlike NA, Nam has no GPR109A agonist activity [80], thus escaping the prostaglandin-mediated vasodilatory side effects. Yet, at high doses Nam has a toxic potential (particularly hepatotoxicity), raising health concerns [83] and, as well as with long-term use, can cause negative feedback to inhibit sirtuins [84, 85].
B. NR and NMN
NR has been recognized since the 1950s as an NAD+ precursor in bacteria that lack the enzymes of the de novo and Preiss–Handler pathways [86–88]. This changed in 2004, when Bieganowski and Brenner [32] detected the presence of NR in milk and identified two human NRK enzymes capable of synthesizing NAD+ from NR. Subsequent human and animal studies confirmed that NR can increase intracellular NAD+ in a dose-dependent fashion [34, 89, 90]. Likewise, NMN is an intermediate in the NAD+ salvage pathway. Although less studied than NR, several studies proved that NMN increases NAD+ levels in vitro and in vivo [33, 56, 91–93]. Several recent studies using NR and NMN have attracted major research interest and are discussed later.
4. NAD+ Biosynthesis Augmentation
Several AMPK and NAMPT activators have been studied. Resveratrol is a nonflavonoid polyphenol that is present in red grapes, wine, and pomegranates; activates AMPK and SIRT1; and improves metabolic health status in humans [94–98]. However, conflicting outcomes from clinical studies have questioned the efficacy of resveratrol in treating human metabolic disease [99]. Nonetheless, it remains a compound of substantial interest to many [100].
Various AMPK activators exist [101]. Among them is metformin, which was introduced in the 1950s to treat diabetes, with a multitude of favorable metabolic outcomes that rely on AMPK [102]. Cantó et al. [45] reported that the AMPK activators metformin and 5-aminoimidazole-4-carboxamide ribonucleotide, increase NAD+ and sirtuin activity, thereby regulating energy expenditure .
Other compounds have also been reported to increase NAMPT activity. P7C3, a neuroprotective chemical that enhances neuron formation, can bind NAMPT and increase NAD+ levels [103–105]. Likewise, the antioxidant troxerutin, a trihydroxyethylated derivative of the natural bioflavonoid rutin, markedly increased NAD+ levels and potentiated SIRT1 via NAMPT activation and PARP1 inhibition in HFD-treated mouse liver [106]. Remarkably, leucine supplementation in obese mice also increased NAMPT expression and enhanced intracellular NAD+ levels [107]. Moreover, proanthocyanidins, the most abundant flavonoid polyphenols in human diet, can dose-dependently increase NAD+ levels in rat liver via the increased expression of the de novo pathway enzymes [108], and possibly NAMPT [109]. Targeting microRNA, such as antagonizing hepatic miR-34a, has also been reported to increase NAMPT expression and NAD+ and SIRT1 activity in vivo [110].
5. Inhibition of NAD+ Consumers
Inhibiting the nonsirtuin NAD+ consumers also increases NAD+ levels and favors sirtuin activity. Inhibitors of PARPs or CD38 induce NAD+ levels, upregulate sirtuins, and enhance mitochondrial gene expression [67, 68, 89, 111]. PARP inhibitors are effective anticancer agents through DNA damage repair and improved oxidative metabolism (opposing the Warburg effect) in which the NAD+-sirtuin axis may be implicated [112–115]. The first PARP inhibitor, olaparib, is now licensed in the United States and Europe for the treatment of ovarian cancer [116, 117]. Therefore, PARP inhibitors may undergo further studies as NAD+-sparing agents to improve adaptive metabolism [118]. Interestingly, troxerutin and proanthocyanidins also inhibit PARPs in mice, thereby contributing to increased NAD+ [106, 108].
6. Type 2 Diabetes Mellitus
The global burden of obesity, insulin resistance, and type 2 diabetes mellitus (T2DM) continues to limit population health through increased cardiovascular disease risk and premature death [119].
Several studies support the notion that defective mitochondrial structure and function are strongly linked to insulin resistance and T2DM [120–128]. The most described mechanism is via defective mitochondrial fatty acid oxidation and the resultant accumulation of intracellular fatty acid metabolites and reactive oxygen species decreasing insulin sensitivity [129–133]. In addition, perturbed oxidative phosphorylation (OXPHOS) may be a direct cause of insulin resistance [134]. Supporting this, obesity reduces mitochondrial enzymatic activities [135, 136] and engenders metabolic inflexibility [137]; the inability to limit fatty oxidation and switch to carbohydrate oxidation in response to diet (and therefore insulin stimulation) [138–141].
Impaired NAD+-mediated sirtuin signaling is also implicated in insulin resistance and T2DM. In particular, defective SIRT1 activity is thought to be a factor in impaired insulin sensitivity [142–148]. This is endorsed by the finding that metformin acts through hepatic SIRT1 activation as part of its diabetes ameliorating effects [149]; results similarly observed with resveratrol [150].
Lifestyle manipulations, such as CR and exercise, can reverse insulin resistance and T2DM and share common mechanistic pathways of AMPK activation leading to elevated NAMPT-mediated NAD+ generation and SIRT1 activity to enhance mitochondrial function [46, 61, 151, 152]. Corroborating the link to NAD+, adipocyte-specific NAMPT deletion in mice decreased adiponectin production and resulted in severe multiorgan insulin resistance [92]. Aside from insulin sensitization, NAD+ and SIRT1 regulate glucose-stimulated insulin secretion in pancreatic β cells [153–155]. NAMPT inhibition and the lack of SIRT1 resulted in pancreatic β cell dysfunction [93, 156–159]. Interestingly, SIRT1 regulates the key components of the circadian clock, CLOCK and BMAL1 [160, 161], and when circadian misalignment is induced in mice, reduced hepatic BMAL1 and SIRT1 levels and insulin resistance ensue [150].
These lines of evidence suggest that an alternate strategy is to increase the level of NAD+ available to affected cells and tissues. Indeed, the NAD+ precursors used to enhance target tissue NAD+ availability have demonstrated efficacy to improve insulin sensitivity and reduce diabetic burden and associated metabolic derangements in preclinical models [33, 162].
NMN administration restored β cell glucose-stimulated insulin secretion and hepatic and muscle insulin sensitivity in mouse models of induced glucose intolerance [33, 92, 93]. Furthermore, Nam treatment in obese rats with T2DM promoted sirtuin-induced mitochondrial biogenesis and improved insulin sensitivity [82]. Similarly, NR supplementation attenuated HFD-induced obesity in mice, improved insulin sensitivity and glucose tolerance, and ameliorated the adverse lipid profile [54, 162]. Moreover, leucine supplementation in obese mice increased NAD+, mitochondrial biogenesis, insulin sensitivity, and lipid disposal [107].
Thus far, clinical data are limited to acipimox and resveratrol. Acipimox increased tissue insulin sensitivity in T2DM [79, 163–168] and improved β cell function when combined with dapagliflozin [76]. However, the results have been inconsistent at times. For instance, acipimox treatment in obese nondiabetic persons alleviated free fatty acids and fasting glucose with a trend toward reduced fasting insulin and homeostatic model assessment of insulin resistance [77], whereas van de Weijer et al. [78] did not report similar benefits in individuals with T2DM by using euglycemic hyperinsulinemic clamp studies. However, in the later study, this may have been related to the rebound increase in fatty acids after short-term acipimox administration [169]. Similarly, many describe that resveratrol decreases glucose and insulin levels in patients with impaired glucose tolerance and diabetes [95, 96, 170, 171], whereas others have not observed these findings [172]. The conflicting results among these studies may be explained by the heterogeneity in the selection of study population, dose and duration of treatment, and the methods of assessing insulin sensitivity.
7. Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease in the Western world, encompassing the spectrum of liver diseases, including simple steatosis, nonalcoholic steatohepatitis, cirrhosis, liver failure, and hepatocellular carcinoma [173]. Hepatic lipid accumulation, which leads to cellular dysfunction, termed lipotoxicity, forms the basis for the development of NAFLD [174–176]. Consequently, a set of metabolic adaptations supervene, such as increased β oxidation. This adaptation induces metabolic inflexibility and drives the oxidative stress and mitochondrial dysfunction that are apparent in NAFLD [177–180].
Sufficient NAD+ levels are essential for adequate mitochondrial fatty acid oxidation [181, 182], and lipid caloric overload in mice reduces hepatic NAD+ levels and triggers lipotoxicity [183]. Zhou et al. [184] demonstrated that hepatic NAD+ levels decline with age in humans and rodents, which may contribute to NAFLD susceptibility during aging. Likewise, ample evidence suggests that impaired hepatic SIRT1 and SIRT3 signaling contributes to NAFLD [183, 185–188] and that SIRT1 overexpression reverses hepatic steatosis [189, 190]. Stressing the significance of adequate hepatic NAD+ homeostasis, aberrant NAD+ metabolism is also implicated in alcoholic hepatic steatosis [191, 192] and hepatocellular carcinoma [193].
Several strategies targeting NAD+ metabolism to enhance sirtuin signaling have proved beneficial in the context of NAFLD. Nam and resveratrol protected hepatocytes in vitro against palmitate-induced endoplasmic reticulum stress [64, 194]. NR attenuated the severe mitochondrial dysfunction present in fatty liver of mice on HFD via NAD+-mediated sirtuin activation [54, 195]. Remarkably, NR was able to target many of the molecular aspects of NAFLD pathogenesis, including decreasing hepatic expression of inflammatory genes, blood tumor necrosis factor-α levels, and the hepatic infiltration by CD45 leukocytes [196]. PARP inhibition in mice with NAFLD can correct NAD+ deficiency, augmenting mitochondrial function and insulin sensitivity and allaying hepatic lipid accumulation and transaminitis [197]. Considering the current data, and in the absence of licensed therapies for NAFLD, replenishing the hepatic NAD+ pool to activate sirtuins and tackle mitochondrial dysfunction is staged for assessment in human clinical studies.
8. Aging and Metabolic Decline
By the year 2050, it is projected that the US population aged ≥65 years will be 83.7 million [198], with other low-mortality countries displaying similar population proportions [199].
Sarcopenia, Greek for “poverty of flesh,” is a consistent manifestation of aging, associated with frailty, metabolic disease, cardiovascular morbidity and mortality, and substantial health care costs [200, 201]. Needless to say, strategies aimed at treating sarcopenia and age-related diseases are needed.
A decline in NAD+ homeostasis contributes to the aging process [202, 203]. Indeed, NAD+ and sirtuins regulate diverse pathways that control aging and longevity [31, 57, 204–206], converging on the ability to defend mitochondrial function [207]. Certainly, mitochondrial dysfunction and defective cellular energy signaling have emerged as critical in aging and age-related metabolic diseases, such as T2DM, NAFLD, and sarcopenia [55]. Specifically, altered mitochondrial homeostasis, through reduced NAD+ and SIRT1 activity, is advocated as a hallmark of muscle aging [56]. In addition, limiting NAD+ in mouse skeletal muscle induced the loss of muscle mass and function (i.e., sarcopenia) [208].
Age-related decline in NAD+ results from several mechanisms, which include accumulating DNA damage (and, consequently, chronic PARPs activation) [209, 210] and increased expression of CD38, clearing NAD+ and inducing mitochondrial dysfunction [211]. Additionally, chronic inflammation [212], a common feature in aging, reduces NAMPT expression and the ability to regenerate adequate NAD+ in multiple tissues [154].
The potential of NAD+ supplementation to support healthy aging is supported by several recent studies. NAMPT overexpression in aged mice matched the NAD+ levels and muscle phenotype of young mice [208]. Furthermore, SIRT1 overexpressing mice were protected against the age-related development of diabetes and had a lower incidence of cancer [213]. NMN administration in aged mice restored NAD+ levels and the markers of mitochondrial function that decline with age [56].
Looking from a different angle, NR supplementation enhanced the expression of PGC-1α in the brain of a mouse model of Alzheimer’s disease, significantly attenuating the cognitive decline [214]. These findings affirm that decreased NAD+ levels contribute to the aging process and that NAD+ supplementation may prevent and even treat age-related diseases.
9. Discussion and Future Challenges
It is now well established that NAD+ is involved in metabolic regulation via redox and cell signaling reactions and that insufficient NAD+ is linked to a variety of metabolic and age-related diseases. The evidence reviewed here highlights that NAD+ levels can be therapeutically increased to potentiate sirtuins and mitochondrial function. This is a great opportunity in metabolic research that could conceivably lead to clinical utility.
The long-known lipid-lowering effects of NA may, at least partly, be NAD+ mediated. This hypothesis is favored because the half maximal effective concentration for the GPR109A receptor is in the nanomolar range [215, 216]; however, the therapeutic doses of NA are greatly in excess of this amount [71, 217]. Moreover, NR ameliorated hypercholesterolemia in mice without activating the GPR109A receptor [54]. Additionally, the liver lacks GPR109A receptors [218] but expresses liver X receptors, which regulate whole-body lipid homeostasis, that are upregulated by SIRT1 [219].
Although we have described the different pathways to NAD+ biosynthesis, it must be emphasized that not all tissues are capable of converting each precursor to NAD+ with equal efficacy, owing to the differences in the cell- and tissue-specific enzyme expression. For instance, cells must express the kynurenine pathway for de novo NAD+ synthesis, clearly active in the liver and brain [12], and must possess the Preiss–Handler pathway to use NA, which is active in most organs but less prominent in skeletal muscle. In contrast, the salvage pathways are crucial in all tissues to conserve NAD+ sufficiency [220]. Supporting this notion, the recommended daily allowance for NA is in milligrams, whereas an estimated 6 to 9 g of NAD+ are required daily to match turnover [58]. This is facilitated by the high affinity of NAMPT for Nam; thus, even small amounts of Nam are effectively converted to NMN and then NAD+ [221].
In the absence of head-to-head studies comparing the different compounds under defined conditions, it is currently not possible to identify the optimal NAD+ augmenting agent. The ubiquitous expression of NRKs, makes NR a precursor that can affect whole-body metabolism [162]. The inability of NR to activate the GPR109A receptor mitigates the undesirable NA side effects, and, unlike Nam, NR does not inhibit sirtuins. Furthermore, NAD+ generated from NR can target both nuclear and mitochondrial NAD+ pools, activating the respective compartmental sirtuins (i.e., nuclear SIRT1 and mitochondrial SIRT3) [54]. This may be an advantage over other molecules, such as PARP inhibitors, with effects confined to the nucleus [67]. Similar to NR, NMN metabolism into NAD+ is governed by the salvage pathway. However, NMN availability has not been characterized in the diet [93, 222], unlike the naturally available NR.
In major proof-of-concept studies, therapeutically increasing NAD+ has been used to treat mouse models of mitochondrial diseases. Treatment of cytochrome C oxidase deficiency in mice with NR, PARP inhibition, and the AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide reversed the mitochondrial dysfunction and improved muscle performance [223–225], effects attributed to NAD+ and sirtuins activation. Treatment of patients with T2DM by using acipimox resulted in improved skeletal muscle oxidative metabolism and mitochondrial function, measured by high-resolution respirometry [78]. However, this acipimox effect was not observed in obese persons without T2DM when assessed by phosphocreatine recovery magnetic resonance spectroscopy, mitochondrial biogenesis gene expression, and mitochondrial density on electron microscopy [77]. Two differences between these studies may explain the observed discrepancy. First, high-resolution respirometry is the current gold standard for ex vivo assessment of mitochondrial respiration if increased oxidative phosphorylation is the question [226]. Second, whereas mitochondrial dysfunction is evident in patients with T2DM, this is not prominent in obese persons without diabetes. Thus, the effects of NAD+ precursor supplementation may vary depending on the intervention and specific pathophysiologic conditions. Nam acts as an NAD+ precursor, increasing SIRT1 activity (below a threshold of sirtuin inhibition), or, conversely, a SIRT1 inhibitor, depending on the specific pathophysiologic state [84, 85].
We still have a limited understanding of the molecular interconversions of the administered NAD+ precursors. Illustrating this, administered NR is converted to Nam in the circulation before entering the cell [208, 227], whereas NMN is transformed extracellularly into NR, which then enters the cell and converts into NAD+ [227].
Knowledge gaps still persist in the role of sirtuins in different contexts. Some reports suggest that not all beneficial SIRT1 activation is through NAD+ and that cyclic adenosine monophosphate plays a role, independent of NAD+, in low-energy states [228, 229]. Upon pharmacologic NAMPT inhibition, Nam failed to increase NAD+; however, this did not prevent SIRT1 upregulation, which was secondary to Nam-induced increase in intracellular cyclic adenosine monophosphate [64].
An important question is whether amplifying NAD+ and sirtuin activity is always desirable. SIRT1 upregulates T helper 17 cells that contribute to autoimmune disease when hyperactivated [230]. Correspondingly, SIRT1 inhibition supports the development of the regulatory T cells that protect against autoimmunity [231, 232]. Therefore, it is possible that SIRT1 activation places susceptible individuals at increased risk for autoimmune diseases. In the same way, whereas NR supplementation increased muscle stem cell number in aged mice, thereby enhancing mitochondrial function and muscle strength, it reduced the expression of cell senescence and apoptosis markers [233]; the state of senescence is important to protect against carcinogenesis [234]. Also, increased NAMPT expression is reported in some malignancies, calling into question whether increasing NAD+ might support aspects of the tumorigenic process [235].
Given the effect of the NAD+-sirtuin pathway on mitochondrial and metabolic homeostasis, novel supplementation strategies (e.g., using NR or NMN) may be exploited to increase endogenous NAD+ availability in the treatment of metabolic and age-related diseases. This is the time for carefully designed human clinical studies to further examine these compounds before we can propose them as being useful nutraceuticals to counteract metabolic disease.
Acknowledgments
Acknowledgments
Y.S.E. is funded by an MRC Arthritis Research UK Clinical Research Fellowship. G.G.L. is in receipt of a Wellcome Trust Senior Research Fellowship (104612/Z/14/Z).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADP
- adenosine 5′-diphosphate
- AMPK
- adenosine monophosphate-activated protein kinase
- ATP
- adenosine triphosphate
- CR
- caloric restriction
- HFD
- high-fat diet
- NA
- nicotinic acid
- NAD+
- nicotinamide adenine dinucleotide
- NADH
- reduced nicotinamide adenine dinucleotide
- NADPH
- phosphorylated nicotinamide adenine dinucleotide
- NAFLD
- nonalcoholic fatty liver disease
- Nam
- nicotinamide
- NAMPT
- nicotinamide phosphoribosyltransferase
- NMN
- nicotinamide mononucleotide
- NR
- nicotinamide riboside
- NRK
- nicotinamide riboside kinase
- PARP
- poly-adenosine 5′-diphosphate ribose polymerase
- PGC-1α
- peroxisome proliferator-activated receptor γcoactivator-1α
- T2DM
- type 2 diabetes mellitus.
References and Notes
- 1.Berger F, Ramírez-Hernández MH, Ziegler M. The new life of a centenarian: signalling functions of NAD(P). Trends Biochem Sci. 2004;29(3):111–118. [DOI] [PubMed] [Google Scholar]
- 2.Lanska DJ. Historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. Handb Clin Neurol. 2009;95:445–476. doi.org/10.1016/S0072-9752(08)02130-1. [DOI] [PubMed] [Google Scholar]
- 3.Rajakumar K. Pellagra in the United States: a historical perspective. South Med J. 2000;93(3):272–277. [PubMed] [Google Scholar]
- 4.Koehn CJ, Elvehjem CA. Further studies on the concentration of the antipellagra factor. J Biol Chem. 1937;118:693–699. [Google Scholar]
- 5.Elvehjem CA, Madden RJ, Strong FM, Woolley DW. Relation of nicotinic acid and nicotinic acid amide to canine black tongue. J Am Chem Soc. 1937;59(9):1767–1768. [Google Scholar]
- 6.Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31(2):194–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther. 2008;324(3):883–893. [DOI] [PubMed] [Google Scholar]
- 8.Pollak N, Dölle C, Ziegler M. The power to reduce: pyridine nucleotides--small molecules with a multitude of functions. Biochem J. 2007;402(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32(1):12–19. [DOI] [PubMed] [Google Scholar]
- 10.Ruggieri S, Orsomando G, Sorci L, Raffaelli N. Regulation of NAD biosynthetic enzymes modulates NAD-sensing processes to shape mammalian cell physiology under varying biological cues. Biochim Biophys Acta. 2015;1854(9):1138–1149. [DOI] [PubMed] [Google Scholar]
- 11.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004;61(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. [DOI] [PubMed] [Google Scholar]
- 13.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13(7):411–424. [DOI] [PubMed] [Google Scholar]
- 14.Malanga M, Althaus FR. The role of poly(ADP-ribose) in the DNA damage signaling network. Biochem Cell Biol. 2005;83(3):354–364. [DOI] [PubMed] [Google Scholar]
- 15.Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem. 2012;287(38):31633–31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7(2):104–112. [DOI] [PubMed] [Google Scholar]
- 17.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39(5):335–345. [DOI] [PubMed] [Google Scholar]
- 18.Langley B, Sauve A. Sirtuin deacetylases as therapeutic targets in the nervous system. Neurotherapeutics. 2013;10(4):605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebastián C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287(51):42444–42452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava S, Diaz F, Iommarini L, Aure K, Lombes A, Moraes CT. PGC-1α/β induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders. Hum Mol Genet. 2009;18(10):1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434(7029):113–118. [DOI] [PubMed] [Google Scholar]
- 23.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem. 2005;280(16):16456–16460. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. [DOI] [PubMed] [Google Scholar]
- 25.van der Horst A, Burgering BMT. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8(6):440–450. [DOI] [PubMed] [Google Scholar]
- 26.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551–563. [DOI] [PubMed] [Google Scholar]
- 27.Brunet A. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. [DOI] [PubMed] [Google Scholar]
- 28.Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+. J Cell Biol. 2012;199(2):205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai S. A possibility of nutriceuticals as an anti-aging intervention: activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol Res. 2010;62(1):42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. [DOI] [PubMed] [Google Scholar]
- 31.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289(5487):2126–2128. [DOI] [PubMed] [Google Scholar]
- 32.Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117(4):495–502. [DOI] [PubMed] [Google Scholar]
- 33.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trammell SAJ, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dölle C, Skoge RH, Vanlinden MR, Ziegler M. NAD biosynthesis in humans--enzymes, metabolites and therapeutic aspects. Curr Top Med Chem. 2013;13(23):2907–2917. [DOI] [PubMed] [Google Scholar]
- 36.Nishizuka Y, Hayaishi O. Studies on the biosynthesis of nicotinamide adenine dinucleotide. I. Enzymic synthesis of niacin ribonucleotides from 3-hydroxyanthranilic acid in mammalian tissues. J Biol Chem. 1963;238(10):3369–3377. [PubMed] [Google Scholar]
- 37.Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O. Studies on the biosynthesis of nicotinamide adenine dinucleotide. II. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals. J Biol Chem. 1965;240(3):1395–1401. [PubMed] [Google Scholar]
- 38.Chi Y, Sauve AA. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr Opin Clin Nutr Metab Care. 2013;16(6):657–661. [DOI] [PubMed] [Google Scholar]
- 39.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23(9):420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikiforov A, Dölle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286(24):21767–21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754–50763. [DOI] [PubMed] [Google Scholar]
- 42.Kulikova V, Shabalin K, Nerinovski K, Dölle C, Niere M, Yakimov A, Redpath P, Khodorkovskiy M, Migaud ME, Ziegler M, Nikiforov A. Generation, release and uptake of the NAD precursor nicotinic acid riboside by human cells. J Biol Chem. 2015;290(45):27124–27137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99(25):15983–15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14(5):661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Sauve AA. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016;1864(12):1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams GT, Lau KM, Coote JM, Johnstone AP. NAD metabolism and mitogen stimulation of human lymphocytes. Exp Cell Res. 1985;160(2):419–426. [DOI] [PubMed] [Google Scholar]
- 49.Rechsteiner M, Hillyard D, Olivera BM. Turnover at nicotinamide adenine dinucleotide in cultures of human cells. J Cell Physiol. 1976;88(2):207–217. [DOI] [PubMed] [Google Scholar]
- 50.Elliott G, Rechsteiner M. Pyridine nucleotide metabolism in mitotic cells. J Cell Physiol. 1975;86(3 Pt 2, Suppl 2):641–651. [DOI] [PubMed] [Google Scholar]
- 51.Rechsteiner M, Hillyard D, Olivera BM. Magnitude and significance of NAD turnover in human cell line D98/AH2. Nature. 1976;259(5545):695–696. [DOI] [PubMed] [Google Scholar]
- 52.Opitz CA, Heiland I. Dynamics of NAD-metabolism: everything but constant. Biochem Soc Trans. 2015;43(6):1127–1132. [DOI] [PubMed] [Google Scholar]
- 53.Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA. Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res. 2007;85(15):3378–3385. [DOI] [PubMed] [Google Scholar]
- 54.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiarugi A, Dölle C, Felici R, Ziegler M. The NAD metabolome--a key determinant of cancer cell biology. Nat Rev Cancer. 2012;12(11):741–752. [DOI] [PubMed] [Google Scholar]
- 59.Song J, Ke SF, Zhou CC, Zhang SL, Guan YF, Xu TY, Sheng CQ, Wang P, Miao CY. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J Gerontol A Biol Sci Med Sci. 2014;69(1):44–57. [DOI] [PubMed] [Google Scholar]
- 60.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11(3):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finglas PM. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin b6, folate, vitamin B12, pantothenic acid, biotin and choline. Trends Food Sci Technol 2000;11(8):296–297. [PubMed] [Google Scholar]
- 63.Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide riboside is a major NAD+ precursor vitamin in cow milk. J Nutr. 2016;146(5):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Dou X, Li S, Zhang X, Zeng Y, Song Z. Nicotinamide ameliorates palmitate-induced ER stress in hepatocytes via cAMP/PKA/CREB pathway-dependent Sirt1 upregulation. Biochim Biophys Acta. 2015;1853(11):2929–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007;129(3):473–484. [DOI] [PubMed] [Google Scholar]
- 66.Pirinen E, Cantó C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C, Li W, Timmers S, Imhof R, Verbeek J, Pujol A, van Loon B, Viscomi C, Zeviani M, Schrauwen P, Sauve AA, Schoonjans K, Auwerx J. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19(6):1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13(4):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbosa MTP, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21(13):3629–3639. [DOI] [PubMed] [Google Scholar]
- 69.Digby JE, Ruparelia N, Choudhury RP. Niacin in cardiovascular disease: recent preclinical and clinical developments. Arterioscler Thromb Vasc Biol. 2012;32(3):582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54(2):558–559. [DOI] [PubMed] [Google Scholar]
- 71.Zeman M, Vecka M, Perlík F, Hromádka R, Staňková B, Tvrzická E, Žák A. Niacin in the treatment of hyperlipidemias in light of new clinical trials: has niacin lost its place? Med Sci Monit. 2015;21:2156–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol Pharmacol. 2006;70(6):1844–1849. [DOI] [PubMed] [Google Scholar]
- 73.Benyó Z, Gille A, Kero J, Csiky M, Suchánková MC, Nüsing RM, Moers A, Pfeffer K, Offermanns S. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115(12):3634–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paolini JF, Mitchel YB, Reyes R, Kher U, Lai E, Watson DJ, Norquist JM, Meehan AG, Bays HE, Davidson M, Ballantyne CM. Effects of laropiprant on nicotinic acid-induced flushing in patients with dyslipidemia. Am J Cardiol. 2008;101(5):625–630. [DOI] [PubMed] [Google Scholar]
- 75.McKenney J, Bays H, Gleim G, Mitchel Y, Kuznetsova O, Sapre A, Sirah W, Maccubbin D. Safety and tolerability of extended-release niacin-laropiprant: pooled analyses for 11,310 patients in 12 controlled clinical trials. J Clin Lipidol. 2015;9(3):313–325. [DOI] [PubMed] [Google Scholar]
- 76.Merovci A, Abdul-Ghani M, Mari A, Solis-Herrera C, Xiong J, Daniele G, Tripathy D, DeFronzo RA. Effect of dapagliflozin with and without acipimox on insulin sensitivity and insulin secretion in T2DM males. J Clin Endocrinol Metab. 2016;101(3):1249-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makimura H, Stanley TL, Suresh C, De Sousa-Coelho AL, Frontera WR, Syu S, Braun LR, Looby SE, Feldpausch MN, Torriani M, Lee H, Patti ME, Grinspoon SK. Metabolic effects of long-term reduction in free fatty acids with acipimox in obesity: a randomized trial. J Clin Endocrinol Metab. 2016;101(3):1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, Livingstone R, Nowotny P, Sparks LM, Paglialunga S, Szendroedi J, Havekes B, Moullan N, Pirinen E, Hwang JH, Schrauwen-Hinderling VB, Hesselink MK, Auwerx J, Roden M, Schrauwen P. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015;64(4):1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daniele G, Eldor R, Merovci A, Clarke GD, Xiong J, Tripathy D, Taranova A, Abdul-Ghani M, DeFronzo RA. Chronic reduction of plasma free fatty acid improves mitochondrial function and whole-body insulin sensitivity in obese and type 2 diabetic individuals. Diabetes. 2014;63(8):2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pike NB. Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J Clin Invest. 2005;115(12):3400–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem. 1972;247(3):778–783. [PubMed] [Google Scholar]
- 82.Yang SJ, Choi JM, Kim L, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, Park CY. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J Nutr Biochem. 2014;25(1):66–72. [DOI] [PubMed] [Google Scholar]
- 83.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA; European Nicotinamide Diabetes Intervention Trial Group . Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43(11):1337–1345. [DOI] [PubMed] [Google Scholar]
- 84.Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell. 2005;17(6):855–868. [DOI] [PubMed] [Google Scholar]
- 85.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277(47):45099–45107. [DOI] [PubMed] [Google Scholar]
- 86.Gingrich W, Schlenk F. Codehydrogenase I and other pyridinium compounds as V-Factor for Hemophilus influenzae and H. parainfluenzae. J Bacteriol. 1944;47(6):535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leder IG, Handler P. Synthesis of nicotinamide mononucleotide by human erythrocytes in vitro. J Biol Chem. 1951;189(2):889–899. [PubMed] [Google Scholar]
- 88.Shifrine M, Biberstein E. A growth factor for Haemophilus species secreted by a Pseudomonad. Nature. 1960;187(4737):623. [Google Scholar]
- 89.Felici R, Lapucci A, Cavone L, Pratesi S, Berlinguer-Palmini R, Chiarugi A. Pharmacological NAD-boosting strategies improve mitochondrial homeostasis in human complex I-mutant fibroblasts. Mol Pharmacol. 2015;87(6):965–971. [DOI] [PubMed] [Google Scholar]
- 90.Yang T, Chan NYK, Sauve AA. Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. J Med Chem. 2007;50(26):6458–6461. [DOI] [PubMed] [Google Scholar]
- 91.Harlan BA, Pehar M, Sharma DR, Beeson G, Beeson CC, Vargas MR. Enhancing NAD+ salvage pathway reverts the toxicity of primary astrocytes expressing amyotrophic lateral sclerosis-linked mutant superoxide dismutase 1 (SOD1). J Biol Chem. 2016;291(20):10836–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, Qi N, Imai S, Yoshino J. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 2016;16(7):1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6(5):363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. [DOI] [PubMed] [Google Scholar]
- 95.Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY). 2012;4(3):146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67(12):1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev. 2010;131(4):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, Ma QY, Mukhopadhyay P, Nalini N, Pezzuto JM, Richard T, Shukla Y, Surh YJ, Szekeres T, Szkudelski T, Walle T, Wu JM. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6(6):e19881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vang O, Pezzuto JM. Introduction to Resveratrol and Health: 3rd International Conference. Ann N Y Acad Sci. 2015;1348(1):v–vi. [DOI] [PubMed] [Google Scholar]
- 101.Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48(4):e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JR, van Denderen BJ, Kemp BE, Steinberg GR. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19(12):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, Capota M, Britt JK, Kotti T, Ure K, Brat DJ, Williams NS, MacMillan KS, Naidoo J, Melito L, Hsieh J, De Brabander J, Ready JM, McKnight SL. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158(6):1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin TC, Britt JK, De Jesús-Cortés H, Lu Y, Genova RM, Khan MZ, Voorhees JR, Shao J, Katzman AC, Huntington PJ, Wassink C, McDaniel L, Newell EA, Dutca LM, Naidoo J, Cui H, Bassuk AG, Harper MM, McKnight SL, Ready JM, Pieper AA. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Reports. 2014;8(6):1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B. Troxerutin improves hepatic lipid homeostasis by restoring NAD(+)-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem Pharmacol. 2014;91(1):74–86. [DOI] [PubMed] [Google Scholar]
- 107.Li H, Xu M, Lee J, He C, Xie Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am J Physiol Endocrinol Metab. 2012;303(10):E1234–E1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aragonès G, Suárez M, Ardid-Ruiz A, Vinaixa M, Rodríguez MA, Correig X, Arola L, Bladé C. Dietary proanthocyanidins boost hepatic NAD(+) metabolism and SIRT1 expression and activity in a dose-dependent manner in healthy rats. Sci Rep. 2016;6(October 2015):24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ribas-Latre A, Baselga-Escudero L, Casanova E, Arola-Arnal A, Salvadó MJ, Bladé C, Arola L. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Sci Rep. 2015;5(April):10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, Kang Y, Li X, Kemper B, Kemper JK. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12(6):1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62(4):1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39(1):8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuan Y, Liao Y-M, Hsueh C-T, Mirshahidi HR. Novel targeted therapeutics: inhibitors of MDM2, ALK and PARP. J Hematol Oncol. 2011;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng FY, de Bono JS, Rubin MA, Knudsen KE. Chromatin to clinic: the molecular rationale for PARP1 inhibitor function. Mol Cell. 2015;58(6):925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nirsimloo R, Gourley C. The safety and efficacy of olaparib therapy in patients with relapsed ovarian cancer. Expert Rev Anticancer Ther. 2016;16(6):597–603. [DOI] [PubMed] [Google Scholar]
- 117.Tucker H, Charles Z, Robertson J, Adam J. NICE guidance on olaparib for maintenance treatment of patients with relapsed, platinum-sensitive, BRCA mutation-positive ovarian cancer. Lancet Oncol. 2016;17(3):277–278. [DOI] [PubMed] [Google Scholar]
- 118.Doig CL, Lavery GG. PARP inhibitors: staying on target? Cell Chem Biol. 2016;23(12):1442–1443. [DOI] [PubMed] [Google Scholar]
- 119.Jaacks LM, Siegel KR, Gujral UP, Narayan KMV. Type 2 diabetes: a 21st century epidemic. Best Pract Res Clin Endocrinol Metab. 2016;30(3):331–343. [DOI] [PubMed] [Google Scholar]
- 120.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. [DOI] [PubMed] [Google Scholar]
- 121.Parish R, Petersen KF. Mitochondrial dysfunction and type 2 diabetes. Curr Diab Rep. 2005;5(3):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005;2(9):0879–0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57(11):2943–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31(3):364–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54(1):8–14. [DOI] [PubMed] [Google Scholar]
- 127.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52(1):1–8. [DOI] [PubMed] [Google Scholar]
- 128.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Pénicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58(3):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. [DOI] [PubMed] [Google Scholar]
- 131.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5(3):167–179. [DOI] [PubMed] [Google Scholar]
- 132.Chibalin AV, Leng Y, Vieira E, Krook A, Björnholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T, Nylén C, Wang J, Laakso M, Topham MK, Gilbert M, Wallberg-Henriksson H, Zierath JR. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132(3):375–386. [DOI] [PubMed] [Google Scholar]
- 133.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. [DOI] [PubMed] [Google Scholar]
- 134.Gerbitz KD, Gempel K, Brdiczka D. Mitochondria and diabetes. Genetic, biochemical, and clinical implications of the cellular energy circuit. Diabetes. 1996;45(2):113–126. [DOI] [PubMed] [Google Scholar]
- 135.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9(2):273–278. [PubMed] [Google Scholar]
- 136.Simoneau JA, Bouchard C. Skeletal muscle metabolism and body fat content in men and women. Obes Res. 1995;3(1):23–29. [DOI] [PubMed] [Google Scholar]
- 137.Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159(6):1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prior SJ, Ryan AS, Stevenson TG, Goldberg AP. Metabolic inflexibility during submaximal aerobic exercise is associated with glucose intolerance in obese older adults. Obesity (Silver Spring). 2014;22(2):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kelley DE, Goodpaster B, Wing RR, Simoneau J-A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277(6 Pt 1):E1130–E1141. [DOI] [PubMed] [Google Scholar]
- 140.Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51(4):1022–1027. [DOI] [PubMed] [Google Scholar]
- 141.Kotronen A, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia. 2008;51(1):130–138. [DOI] [PubMed] [Google Scholar]
- 142.Wang R, Kim H, Xiao C, Xu X. Hepatic Sirt1 deficiency in mice impairs mTorc2 / Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121(11):4477–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liang F, Chen R, Nakagawa A, Nishizawa M, Tsuda S, Wang H, Koya D. Low-frequency electroacupuncture improves insulin sensitivity in obese diabetic mice through activation of SIRT1/PGC-1α in skeletal muscle. Evidence-based Complement Altern Med. 2011;2011:735297. 10.1155/2011/735297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang H-H, Ma X-J, Wu L-N, Zhao YY, Zhang PY, Zhang YH, Shao MW, Liu F, Li F, Qin GJ. SIRT1 attenuates high glucose-induced insulin resistance via reducing mitochondrial dysfunction in skeletal muscle cells. Exp Biol Med (Maywood). 2015;240(5):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fröjdö S, Durand C, Molin L, Carey AL, El-Osta A, Kingwell BA, Febbraio MA, Solari F, Vidal H, Pirola L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol. 2011;335(2):166–176. [DOI] [PubMed] [Google Scholar]
- 146.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16(2):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rutanen J, Yaluri N, Modi S, Pihlajamäki J, Vänttinen M, Itkonen P, Kainulainen S, Yamamoto H, Lagouge M, Sinclair DA, Elliott P, Westphal C, Auwerx J, Laakso M. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59(4):829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu C, Bai B, Fan P, Cai Y, Huang B, Law IK, Liu L, Xu A, Tung C, Li X, Siu FM, Che CM, Vanhoutte PM, Wang Y. Selective overexpression of human SIRT1 in adipose tissue enhances energy homeostasis and prevents the deterioration of insulin sensitivity with ageing in mice. Am J Transl Res. 2013;5(4):412–426. [PMC free article] [PubMed] [Google Scholar]
- 149.Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205(1):97–106. [DOI] [PubMed] [Google Scholar]
- 150.Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, Wang Y, Hu Y, Wu J, Dai C, Wang H, Tu Y, Peng X, Wang Y, Zhai Q. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology. 2014;59(6):2196–2206. [DOI] [PubMed] [Google Scholar]
- 151.Meex RCR, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59(3):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phielix E, Meex R, Moonen-Kornips E, Hesselink MKC, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53(8):1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kover K, Tong PY, Watkins D, Clements M, Stehno-Bittel L, Novikova L, Bittel D, Kibiryeva N, Stuhlsatz J, Yan Y, Ye SQ, Moore WV. Expression and regulation of nampt in human islets. PLoS One. 2013;8(3):e58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab. 2013;15(Suppl 3):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2(2):105–117. [DOI] [PubMed] [Google Scholar]
- 156.Luu L, Dai FF, Prentice KJ, Huang X, Hardy AB, Hansen JB, Liu Y, Joseph JW, Wheeler MB. The loss of Sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia. 2013;56(9):2010–2020. [DOI] [PubMed] [Google Scholar]
- 157.Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J. 2011;278(7):1167–1174. [DOI] [PubMed] [Google Scholar]
- 158.do Amaral MEC, Ueno M, Oliveira CAM, Borsonello NC, Vanzela EC, Ribeiro RA, Alves PL, Barbosa HC, Carneiro EM, Boschero AC. Reduced expression of SIRT1 is associated with diminished glucose-induced insulin secretion in islets from calorie-restricted rats. J Nutr Biochem. 2011;22(6):554–559. [DOI] [PubMed] [Google Scholar]
- 159.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. [DOI] [PubMed] [Google Scholar]
- 162.Trammell SAJ, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci Rep. 2016;6(May):26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005;54(11):3148–3153. [DOI] [PubMed] [Google Scholar]
- 164.Worm D, Henriksen JE, Vaag A, Thye-Rønn P, Melander A, Beck-Nielsen H. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab. 1994;78(3):717–721. [DOI] [PubMed] [Google Scholar]
- 165.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48(9):1836–1841. [DOI] [PubMed] [Google Scholar]
- 166.Liang H, Tantiwong P, Sriwijitkamol A, Shanmugasundaram K, Mohan S, Espinoza S, Defronzo RA, Dubé JJ, Musi N. Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects. J Physiol. 2013;591(11):2897–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vaag A, Skött P, Damsbo P, Gall M-A, Richter EA, Beck-Nielsen H. Effect of the antilipolytic nicotinic acid analogue acipimox on whole-body and skeletal muscle glucose metabolism in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1991;88(4):1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fulcher GR, Catalano C, Walker M, Farrer M, Thow J, Whately-Smith CR, Alberti KG. A double blind study of the effect of acipimox on serum lipids, blood glucose control and insulin action in non-obese patients with type 2 diabetes mellitus. Diabet Med. 1992;9(10):908–914. [DOI] [PubMed] [Google Scholar]
- 169.Saloranta C, Taskinen MR, Widen E, Härkönen M, Melander A, Groop L. Metabolic consequences of sustained suppression of free fatty acids by acipimox in patients with NIDDM. Diabetes. 1993;42(11):1559–1566. [DOI] [PubMed] [Google Scholar]
- 170.Pérez-Rubio KG, González-Ortiz M, Martínez-Abundis E. Robles-Cervantes J a, Espinel-Bermúdez MC. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2013;11(5):366–369. [DOI] [PubMed] [Google Scholar]
- 171.Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, Mészáros LG, Sümegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106(3):383–389. [DOI] [PubMed] [Google Scholar]
- 172.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H , Møller N, Jessen N, Pedersen SB, Jørgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62(4):1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–530.e1, quiz e60. [DOI] [PubMed] [Google Scholar]
- 174.Leamy AK, Egnatchik RA, Shiota M, Ivanova PT, Myers DS, Brown HA, Young JD. Enhanced synthesis of saturated phospholipids is associated with ER stress and lipotoxicity in palmitate treated hepatic cells. J Lipid Res. 2014;55(7):1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28(Suppl 1):68–76. [DOI] [PubMed] [Google Scholar]
- 176.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21(5):739–746. [DOI] [PubMed] [Google Scholar]
- 178.Serviddio G, Bellanti F, Tamborra R, Rollo T, Romano AD, Giudetti AM, Capitanio N, Petrella A, Vendemiale G, Altomare E. Alterations of hepatic ATP homeostasis and respiratory chain during development of non-alcoholic steatohepatitis in a rodent model. Eur J Clin Invest. 2008;38(4):245–252. [DOI] [PubMed] [Google Scholar]
- 179.Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38(4):999–1007. [DOI] [PubMed] [Google Scholar]
- 180.Lee S, Rivera-Vega M, Alsayed HMAA, Boesch C, Libman I. Metabolic inflexibility and insulin resistance in obese adolescents with non-alcoholic fatty liver disease. Pediatr Diabetes. 2015;16(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, Yoo SK, Park MK, Kwak TH, Kho YL, Han J, Choi HS, Lee SH, Kim JM, Lee I, Kyung T, Jang C, Chung J, Kweon GR, Shong M. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58(4):965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15(11):1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, Watson PA, Birdsey N, Bao J, Gius D, Sack MN, Jing E, Kahn CR, Friedman JE, Jonscher KR. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433(3):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou C-C, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF, Wang P, Miao CY. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173(15):2352–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15(5):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xu F, Gao Z, Zhang J, Rivera CA, Yin J, Weng J, Ye J. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: a role of lipid mobilization and inflammation. Endocrinology. 2010;151(6):2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L, Zang M. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146(2):539–49.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105(28):9793–9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Marmier S, Dentin R, Daujat-Chavanieu M, Guillou H, Bertrand-Michel J, Gerbal-Chaloin S, Girard J, Lotersztajn S, Postic C. Novel role for carbohydrate responsive element binding protein in the control of ethanol metabolism and susceptibility to binge drinking. Hepatology. 2015;62(4):1086–1100. [DOI] [PubMed] [Google Scholar]
- 192.Yin H, Hu M, Liang X, Ajmo JM, Li X, Bataller R, Odena G, Stevens SM Jr, You M. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology. 2014;146(3):801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tummala KS, Gomes AL, Yilmaz M, Graña O, Bakiri L, Ruppen I, Ximénez-Embún P, Sheshappanavar V, Rodriguez-Justo M, Pisano DG, Wagner EF, Djouder N. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26(6):826–839. [DOI] [PubMed] [Google Scholar]
- 194.Jung TW, Lee KT, Lee MW, Ka KH. SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem Biophys Res Commun. 2012;422(2):229–232. [DOI] [PubMed] [Google Scholar]
- 195.Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, Kim B, Park YK, Piersigilli A, Pham TX, Yang Y, Ku CS, Koo SI, Fomitchova A, Cantó C, Schoonjans K, Sauve AA, Lee JY, Auwerx J. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63(4):1190–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. [DOI] [PubMed] [Google Scholar]
- 197.Gariani K, Ryu D, Menzies K, Yi H-S, Stein S, Zhang H, Perino A, Lemos V, Katsyuba E, Jha P, Vijgen S, Rubbia-Brandt L, Kim YK, Kim JT, Kim KS, Shong M, Schoonjans K, Auwerx J. Inhibiting poly-ADP ribosylation increases fatty acid oxidation and protects against fatty liver disease. J Hepatol. 2016;66(1):132–141. [DOI] [PubMed] [Google Scholar]
- 198.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. Current Population Reports. May 2014.
- 199. Office for National Statistics. Population ageing in the United Kingdom, its constituent countries and the European Union. 2012;(March):1-12. doi:10.1057/pt.2010.7.
- 200.Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sousa AS, Guerra RS, Fonseca I, Pichel F, Ferreira S, Amaral TF. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr. 2015;2016:1–6. [DOI] [PubMed] [Google Scholar]
- 202.Chini CCS, Tarragó MG, Chini EN. NAD and the aging process: Role in life, death and everything in between [published online ahead of print November 5, 2016]. Mol Cell Endocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Imai S. Dissecting systemic control of metabolism and aging in the NAD world: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett. 2011;585(11):1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. [DOI] [PubMed] [Google Scholar]
- 206.Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, Price NL, Schmeisser S, Schuster S, Pfeiffer AF, Guthke R, Platzer M, Hoppe T, Cohen HY, Zarse K, Sinclair DA, Ristow M. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013;9(11):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Frederick DW, Loro E, Liu L, Davila A Jr, Chellappa K, Silverman IM, Quinn WJ III, Gosai SJ, Tichy ED, Davis JG, Mourkioti F, Gregory BD, Dellinger RW, Redpath P, Migaud ME, Nakamaru-Ogiso E, Rabinowitz JD, Khurana TS, Baur JA. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016;24(2):269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7(7):e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Veith S, Mangerich A.. RecQ helicases and PARP1 team up in maintaining genome integrity. Ageing Res Rev. 2015;23(PA):12–28. [DOI] [PubMed] [Google Scholar]
- 211.Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016;23(6):1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34(6):1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim HY, Jadhav VB, Jeong DY, Park WK, Song JH, Lee S, Cho H. Discovery of 4-(phenyl)thio-1H-pyrazole derivatives as agonists of GPR109A, a high affinity niacin receptor. Arch Pharm Res. 2015;38(6):1019–1032. [DOI] [PubMed] [Google Scholar]
- 216.Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, Brown AJ, Dowell SJ, Szekeres PG, Hassall DG, Marshall FH, Wilson S, Pike NB. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278(11):9869–9874. [DOI] [PubMed] [Google Scholar]
- 217.Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol. 2008;48:79–106. [DOI] [PubMed] [Google Scholar]
- 218.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9(3):352–355. [DOI] [PubMed] [Google Scholar]
- 219.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28(1):91–106. [DOI] [PubMed] [Google Scholar]
- 220.Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol. 2015;50(4):284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Burgos ES, Schramm VL. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry. 2008;47(42):11086–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hara N, Yamada K, Shibata T, Osago H, Tsuchiya M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS One. 2011;6(8):e22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014;6(6):721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, Viscomi C, Zeviani M. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19(6):1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, Schon EA, Lamperti C, Zeviani M. In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell Metab. 2011;14(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ryan TE, Brophy P, Lin C-T, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 2014;592(15):3231–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ratajczak J, Joffraud M, Trammell SAJ, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, Auwerx J, Yanes O, Brenner C, Cantó C. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun. 2016;7:13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Noriega LG, Feige JN, Cantó C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12(10):1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gerhart-Hines Z, Dominy JE Jr, Blättler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol Cell. 2011;44(6):851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lim HW, Kang SG, Ryu JK, Schilling B, Fei M, Lee IS, Kehasse A, Shirakawa K, Yokoyama M, Schnölzer M, Kasler HG, Kwon HS, Gibson BW, Sato H, Akassoglou K, Xiao C, Littman DR, Ott M, Verdin E. SIRT1 deacetylates RORγt and enhances Th17 cell generation. J Exp Med. 2015;212(5):607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kwon H-S, Lim HW, Wu J, Schnölzer M, Verdin E, Ott M. Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J Immunol. 2012;188(6):2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, Prakken BJ, Coffer PJ. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115(5):965–974. [DOI] [PubMed] [Google Scholar]
- 233.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle E, Lutolf M, Aebersold R, Schoonjans K, Menzies K, Auwerx J. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;1436–1443. [DOI] [PubMed] [Google Scholar]
- 234.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. [DOI] [PubMed] [Google Scholar]
- 235.Shackelford RE, Mayhall K, Maxwell NM, Kandil E, Coppola D. Nicotinamide phosphoribosyltransferase in malignancy: a review. Genes Cancer. 2013;4(11-12):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]