Abstract
Context:
Congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency (CAH21) is most often diagnosed by newborn screening. The classic parameter studied is 17-hydroxy-progesterone, but the positive predictive value for the diagnosis of CAH is low in full-term newborns and even lower in preterm newborns.
Objective:
To evaluate the diagnostic utility of simultaneously quantifying a large number of steroids by using liquid chromatography/tandem mass spectrometry (LC-MS/MS) from a small serum volume in patients with CAH, particularly during the neonatal period.
Setting and participants:
LC-MS/MS was applied to sera from patients with CAH who had a classic form (n = 48) and rare forms (n = 2) of 21-hydroxylase deficiency, normal preterm (n = 10) and normal full-term (n = 20) neonates, and young patients without CAH (non-CAH; n = 149) but with various other diseases (delayed or advanced puberty, hirsutism, pubarche, adrenarche, simple growth retardation).
Methods:
Sixteen steroids (glucocorticoids, mineralocorticoids, androgens, Δ5-steroids) were analyzed in 150 µL of serum by LC-MS/MS.
Results:
An LC-MS/MS serum steroid profile was developed and validated to provide a reliable etiologic diagnosis of CAH. The serum levels of 17OH-progesterone and 21 deoxycortisol in non-CAH are reported, along with the rarely assayed 21-deoxycorticorticosterone and 11β hydroxy Δ4-androstenedione, which will aid in the diagnosis of CAH21. In addition, serum levels of mineralocorticoids, androgens, and Δ5-steroids allowed investigation of other forms of CAH.
Conclusion:
This steroid LC-MS/MS approach on a small serum volume is well suited for pediatrics, particularly neonatal medical practice, to aid in the diagnosis and monitoring of various forms of CAH.
Keywords: LC-MS/MS, steroids, congenital adrenal hyperplasia, 21 deoxycortisol, 21 deoxycorticosterone
Liquid chromatography/tandem mass spectometry performed on a low serum volume sample is well suited to pediatrics, particularly neonatal medical practice, to aid in the diagnosis and monitoring of various forms of congenital adrenal hyperplasia.
Recent progress in instrumentation, including liquid chromatography/tandem mass spectrometry (LC-MS/MS), has made it possible to simultaneously assay multiple steroids [1]. Two decades ago, we described the simultaneous assay of eight serum steroids [11 deoxycortisol (11DF), 21 deoxycortisol (21DF), 11β hydroxy Δ4-androstenedione (11βOHA), Δ4-androstenedione (A), testosterone (T), 17 OH progesterone (17OHP), dehydroepiandrosterone (DHEA), and 17 OH pregnenolone (17OHPreg)] by using radioimmunoassay (RIA) technology after celite chromatographic purification of 1 to 2 mL of extracted serum to study hyperandrogeny in women [2].
Here, we assayed eight additional steroids [aldosterone (Aldo), cortisone (E), cortisol (F), corticosterone (B), 11 deoxycorticosterone (DOC), 21 deoxycorticosterone (21DB), progesterone (P), and pregnenolone (Preg)] by LC-MS/MS, as well as the eight previously assayed by RIA. We evaluated what implementation of this LC-MS/MS method could bring to the diagnosis and therapeutic follow-up of congenital enzymatic adrenal deficiencies [congenital adrenal hyperplasia (CAH)] to clinical practice, particularly in pediatrics. A simultaneous LC-MS/MS profile that includes 21DF, 21DB, DOC, B, Aldo, , and Δ5 steroids (which are rarely routinely measured) could provide better specificity to CAH screening at birth.
1. Materials
A. High-Performance Liquid Chromatography
To perform high-performance liquid chromatography, Shimadzu Nexera XR system (Shimazu France, Marne la Vallee, France) and a Coreshell C18 column (Kinetex, 2.6 µm 100 Å 100 × 2.1 mm; Phenomenex, Le Pecq, France) were used.
B. Tandem Mass Spectrometer
A triple quadripole mass spectrometer (Triple Quad 6500, ABSciex, Foster City, CA) was used.
C. Extraction System
Positive pressure manifold (Pressure+) and evaporation system (TurboVap LV) were purchased from Biotage (Uppsala, Sweden).
D. Supported Liquid Extraction Columns
Isolute SLE+ (Biotage, Uppsala, Sweden) was used, with 400-µL or 1-mL columns.
E. Steroids
Products from Steraloids (Biovalley, Marne La Vallée, France) were used for calibrator preparation.
F. Stable Isotope–Labeled Compounds
The following were purchased from CDN Isotopes (Pointe-Claire, QC, Canada): aldosterone 2,2,4,6,6,19,19-d7; cortisol-9,11,12,12-d4; 11β-OH-Δ4-androstenedione-d7; 21-deoxycortisol-2,2,4,6,6,21,21,21-d8; corticosterone-2,2,4,6,6,17α,21,21-d8; 11-deoxycortisol-21,21-d2; Δ4-androsten-3,17-dione-2,2,4,6,6,16,16-d7; 11-deoxycorticosterone-2,2,4,6,6,17α,21,21-d8; testosterone-2,2,4,6,6-d5; 17-α-hydroxyprogestérone-2,2,4,6,6,21,21,21-d8; progesterone-2,2,4,6,6,17α,21,21,21-d9; and dehydroepiandrosterone-2,2,3,4,4,6-d6. 17α-Hydroxypregnenolone-20,21-13C2-16,16-d2 and pregnenolone-20,21-13C2-16,16-d2 were purchased from Cambridge Isotope Laboratories (Andover, MA).
G. Solvents and Chemicals
LC-MS grade methanol and dichloromethane were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultra-pure water was produced with Millipore System Charcoal dextran coated (Milli-Q Integral) purchased from Merck Millipore (Saint-Quentin-en-Yvelines, France).
H. Certified Reference Material
Aldo, F, 11DF, T, and 17OHP (Cerilliant) were purchased from Sigma-Aldrich. Each ampule containing 1 mg steroid in 1 mL organic solvent was diluted in 100 mL methanol before being assayed. Steroid-free human serum was purchased from BioMérieux (Marcy l’Etoile, France).
I. Standard Solutions and Calibrators
Methanol solutions of steroids were stored at −20°C. Eight dilutions of a mixture of the 16 steroids were prepared by diluting them into steroid-free serum for the establishment of standard curves. They were stored at −20°C and used within 3 months.
J. Internal Standards
An internal standard mix was prepared in water containing the following (expressed in ng/mL): Aldo-d7 (5), E-d2 (33), F-d4 (66), 11βOHA-d7 (16), 21DF-d8 (50), B-d8 (25), 11DF-d8 (25), A-d7 (5), DOC-d8 (1), T-d5 (2), 17OHP-d8 (10), DHEA-d6 (10), 17 OHPreg-13C2 d2 (10), P-d9 (10), and Preg-13C2 d2 (10). Deuterated internal standards in a methanol solution were separated into aliquots and stored at −20°C and used within 3 months to prevent the loss of deuterium via exchange with hydrogen.
K. Quality Control Serum
We prepared quality control (QC) serum by spiking steroid-free human serum with low, medium, and high amounts of steroids because adequate QC material was not commercially available. QC serum was stored at −20°C and used within 3 months.
2. Methods
A mixture of the deuterated internal standard (150 µL) was added in the same run to 150 µL calibrators, QC serum, or patient serum. The solution was mixed and left standing for 5 minutes and then loaded onto an Isolute SLE+ 0.4 mL cartridge (Biotage) [3–7]. The samples were allowed to adsorb for 5 minutes before elution of the steroids by the addition of 2 × 0.9 mL methylene chloride. The eluates, which contained the nonconjugated steroids, were evaporated to dryness and reconstituted to 150 µL in methanol/water (50/50, volume-to-volume ratio).
Steroids were chromatographically separated by high-performance liquid chromatography, at 45°C, at a flow rate of 0.4 mL/min, by using a gradient from 80% phase A (water + 0.05% formic acid) and 20% phase B (methanol) to 10% phase A and 90% phase B over 10 minutes.
Detection was performed by atmospheric pressure chemical ionization operating in positive mode for all metabolites except aldosterone, which was analyzed in negative mode. The MS parameters, including declustering potential (volts), collision energy (electron-volts), and collision cell exit potential (volts) were optimized to achieve maximum sensitivity (Supplemental Table 1 (94KB, docx) ). The source parameters were as follows: atmospheric pressure chemical ionization voltage, 5500 V (−4500 V for aldosterone); ion source temperature, 500°C; curtain gas, 35 (arbitrary unit); collision dissociation gas, 12 (arbitrary unit); ion source gas, 1 (40 psi); and gas, 2 (50 psi). Selection of the quantifier and qualifier ions was obtained by infusion of the analytes and identified in MS2 mode (Supplemental Table 1 (94KB, docx) ).
After acquisition, the LC-MS/MS data were analyzed by using MultiQuant software (version 3.0) with built-in queries or quality control rules allowing us to set compound-specific criteria for flagging outlier results. Flagging criteria included accuracies for standards and QCs, quantifier ion/qualifier ion ratios, and lower/upper calculated concentration limits.
For each calibration curve, a regression line was calculated by using least-squares method (1/x).
A. Validation
The correlation coefficient was >0.999 for each steroid standard curve. This was considered acceptable because back-calculations of 75% of the concentrations of nonzero standards were within 15% of the nominal concentrations except at the lower limit of quantitation (LLOQ), where 20% is considered acceptable [8].
The LLOQ, lower limit of detection, and upper limits of quantification were established according to established guidelines [9] and are reported in Supplemental Fig. 1 (1MB, tif) .
Precision was evaluated from the analysis of 10 replicates for the three steroid growing levels in the QC serum. All QC interassay values varied by <10% (Supplemental Table 2 (94KB, docx) ).
Accuracy was studied by using several methods depending on available materials. The accuracies were between 85% and 115% relative to nominal control serum values. The biases compared with certified reference material analysis were <6% relative to theoretical values (Table 1). Data from a French external QC program (Probioqual, Lyon, France) (Table 2) show that our results are coherent with those reported by other laboratories using LC-MS/MS or RIA. However, results of the 17OHP assay (Table 2) showed levels of 6.93 ng/mL for our method compared with 6.47 ng/mL for LC-MS/MS in other laboratories and 5.35 ng/mL for all methods (of which 95% were RIA). These discrepancies are likely due to a problem of calibration of the RIA kits.
Table 1.
Certified Reference Material Concentrations Measured by Using LC-MS/MS Method
|
Aldo (ng/mL) |
F (ng/mL) |
11DF (ng/mL) |
T (ng/mL) |
17OHP (ng/mL) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Theoretical | Found | Theoretical | Found | Theoretical | Found | Theoretical | Found | Theoretical | Found |
| 10.0 | 10.5 | 100 | 102.9 | 10.0 | 10.2 | 10 | 9.8 | 100.0 | 101.1 |
| 0.50 | 0.49 | 50.0 | 50.8 | 5.0 | 5.1 | 5.0 | 5.01 | 10.0 | 9.84 |
| 0.10 | 0.098 | 25.0 | 25.9 | 2.50 | 2.45 | 2.50 | 2.65 | 2.50 | 2.35 |
Table 2.
Results From the French External Quality Assessment Program (External Quality Control Probioqual, Lyon, France: From Two Samples of Year 2016)
| Steroids |
Current Method |
LC-MS/MS (Means) |
All Methods (Means) |
|||
|---|---|---|---|---|---|---|
| ng/mL | nmol/L | ng/mL | nmol/L | ng/mL | nmol/L | |
| T | 2.15 | 7.48 | 2.14 | 7.46 | 2.25 | 7.83 |
| 2.21 | 7.69 | 2.29 | 7.97 | 2.30 | 8.01 | |
| Α | 2.86 | 10.60 | 3.23 | 11.32 | 4.06 | 14.22 |
| 0.32 | 1.13 | 0.34 | 1.20 | 0.70 | 2.46 | |
| 17-OHP | 6.83 | 20.68 | 6.47 | 19.06 | 5.35 | 16.02 |
| 0.57 | 1.75 | 0.56 | 1.71 | 0.62 | 1.89 | |
| Aldo | 0.320 | 0.809 | 0.335 | 0.932 | 0.302 | 0.843 |
| 0.086 | 0.240 | 0.082 | 0.230 | 0.077 | 0.215 | |
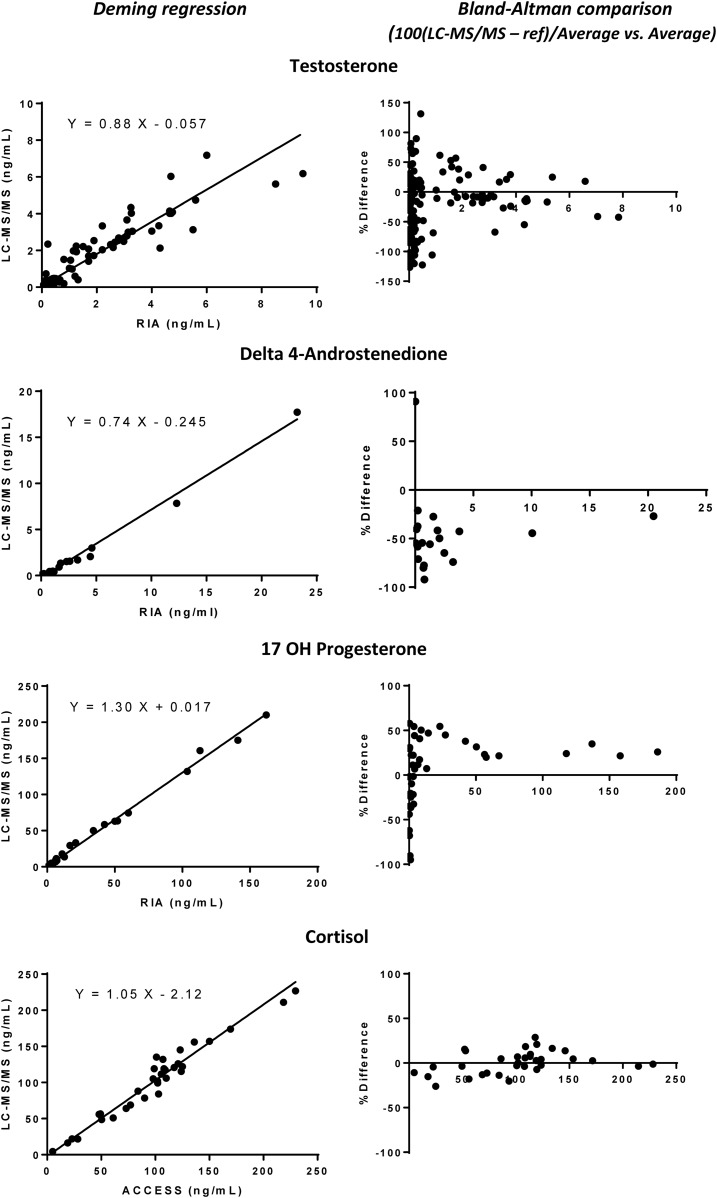
Comparisons of immunoassay and LC-MS/MS results of non-CAH and CAH serum by Deming regression and Bland–Altman analysis (Fig. 1) showed that the cortisol immunoassay gave results almost identical to those of LC-MS/MS. The values for T by RIA (DPC, CA, USA) were higher than by LC-MS/MS, and those of A by RIA (DSL) were considerably higher than by LC-MS/MS, whereas the results for 17OHP by LC-MS/MS were 20% to 30% higher than by RIA. This last discrepancy is in accordance with the results of the French external QC program (see above). However, 17OHP levels measured in preterm and full-term newborns were substantially higher (twofold to fourfold) by RIA than by LC-MS/MS (Table 3). In addition, serum T levels in preterm and full-term newborns were much higher by RIA than by LC-MS/MS (Supplemental Fig. 2 (72.5KB, tiff) ).
Figure 1.
Comparison of RIA and LC-MS/MS values for T, A, 17OHP, and F in serum obtained from patients age >10 days (non-CAH and CAH21). Deeming regression and Bland–Altman plots were used.
Table 3.
Serum Levels of 17OHP, DHEA, and 17OHPreg Assayed by LC-MS/MS and 17OHP Assayed by RIA in 21 Neonates for Neonatal Endocrine Exploration
| Patient No. | Diagnosis | Sex | Age (d) | 17OHP: RIA (ng/mL) | 17OHP: LC-MS (ng/mL) | DHEA: LC-MS (ng/mL) | 17OHPreg: LC-MS (ng/mL) | 17OHP: RIA Reference Values (According to Age) |
|---|---|---|---|---|---|---|---|---|
| Preterm neonates (< 37 weeks of amenorrhea) | ||||||||
| 1 | Mild clitomegaly | F | 6 | 5.5 | 1.3 | 6.3 | 4.4 | 0.3–8 |
| 2 | Neonatal screeninga | M | 7 | 10.8 | 2.4 | 26.9 | 25.1 | 0.3–8 |
| Second follow-up | M | 12 | 9.6 | 3.8 | 14.3 | 13.2 | 0.3–8 | |
| Third follow-up | M | 40 | 8.8 | 1.5 | 25.2 | 24.1 | 0.3–4 | |
| 3 | Temporary digestive disordersa,b | F | 55 | 12.7 | 3.2 | 16.2 | 17.6 | 0.3–4 |
| Second follow-up | F | 60 | 10.2 | ND | ND | ND | 0.3–4 | |
| Third follow-up | F | 90 | 2.95 | ND | ND | ND | 0.2–1 | |
| 4 | Neonatal screening | M | 11 | 5.4 | 1.9 | 22.8 | 22.4 | 0.3–8 |
| 5 | Neonatal screening + severe hypospadias + micropenisb | M | 50 | 6.9 | 3.1 | 17.1 | 20.2 | 0.3–4 |
| 6 | Neonatal screening | M | 10 | 9.1 | 2.1 | 26.1 | 27.1 | 0.3–8 |
| 7 | Neonatal screening | M | 7 | 6.5 | 2.4 | 33.1 | 35.6 | 0.3–8 |
| 8 | Intensive care for enterocolitis | F | 29 | 5.6 | 3.2 | 27.7 | 31.9 | 0.3–8 |
| 9 | Neonatal screeningb | F | 33 | 7.7 | 1.3 | 7.9 | 12.1 | 0.3–6 |
| 10 | Neonatal screening | M | 9 | 6.8 | 1.4 | 16.1 | 16.4 | 0.3–8 |
| Full-term neonates (> 37 weeks of amenorrhea) | ||||||||
| 11 | Tachycardia, diarrhea, vomiting, anorexia | M | 7 | 4.4 | 2.7 | 8.4 | 9.5 | 0.3–8 |
| 12 | Hypotonia | M | 6 | 1.4 | 0.2 | 0.9 | 0.4 | 0.3–8 |
| 13 | Preeclampsia + mild clitomegaly | F | 11 | 7.4 | 2.2 | 8.3 | 7.2 | 0.3–8 |
| 14 | Neonatal screening | F | 7 | 3.5 | 1 | 5.8 | 6.4 | 0.3–8 |
| 15 | Neonatal screening + hypospadias | M | 8 | 6.8 | 2.1 | 4.1 | 8.5 | 0.3–8 |
| 16 | Twin of 15+ hypospadias | M | 8 | 6.1 | 1.7 | 10.4 | 13.9 | 0.3–8 |
| 17 | Neonatal screening | M | 8 | 5.9 | 2.6 | 7.9 | 10.8 | 0.3–8 |
| 18 | Neonatal screening | M | 11 | 6.6 | 2.8 | 47.1 | 41.2 | 0.3–8 |
| 19 | Neonatal screening | F | 5 | 4.2 | 2.4 | 15.1 | 10.8 | 0.3–8 |
| 20 | Neonatal screening | M | 10 | 3.4 | 1.6 | 5.3 | 6.9 | 0.3–8 |
| 21 | Neonatal screening | M | 8 | 9.1 | 1.7 | 8.1 | 18.5 | 0.3–8 |
The 17OHP RIA values giving false-positives results are marked in bold (corresponding to 6 of the 21 patients).
Abbreviations: F, female; M, male; ND, not determined.
Neonatal screening = values of neonatal screening above the normal reference values according to the gestational age (weeks of amenorrhea).
Preterm <33 weeks of amenorrhea (for these older preterm infants, the decision tree of the CAH screening federation (Fédération Parisienne pour le Dépistage et la Prévention des Handicaps de l’Enfant, Hôpital Necker Enfants Malades, Paris) is to carry out screening control after the corrected term between 36 and 38 weeks).
Extracted serum volumes were 150 µL in all experiments. However, in cases of very low Aldo, 17OHPreg, and Preg concentrations, larger samples (450 µL) were re-extracted to improve the LLOQ. DHEA, 17OHPreg, and Preg were unstable in serum samples at laboratory and refrigerator temperature. All serum samples were, therefore, stored at −20°C until analysis [10].
The groups of isobaric steroids were well separated: mass-to-charge ratio of 346 in 4.1, 4.43, and 4.69 minutes for 21DF, B, 11DF, respectively, and mass-to-charge ratio of 330 in 5.72 and 6.24 minutes for DOC and 17OHP, respectively. DOC and 21DB, with close retention times of 5.72 and 5.84 minutes, were selectively assayed because of the choice of different transitions: 109.1 and 97.1 for DOC and 121.2 and 295.2 for 21DB (Supplemental Fig. 3 (1.4MB, tiff) ). We found no matrix effect [11] for the three growing concentrations of the 16 steroids in the QC serum (Supplemental Table 3 (94KB, docx) ). Recoveries were between 88.7% and 116.2%.
B. Population Studied
We systematically applied this LC-MS/MS method to analyze the serum of patients consulting for hormonal investigations between January 2014 and December 2015, divided into four groups: preterm newborns (pregnancy <37 weeks of amenorrhea; n = 10), full-term newborns age <10 days (pregnancy >37 weeks of amenorrhea; n = 20), patients ranging in age from 10 days old to 18 years old without adrenal enzymatic deficiency (non-CAH; n = 149) followed for various conditions (delayed or advanced puberty, hirsutism, pubarche, adrenarche, simple growth retardation), and children evaluated for diagnosis of or follow-up for 21-hydroxylase deficiencies (CAH21; n = 48), as well as one child with17-hydroxylase deficiency and one with 3β-hydroxysteroid dehydrogenase type 2 deficiency.
The treatment of CAH21 included glucocorticoid and mineralocorticoid treatments. Glucocorticoid substitution consisted of oral administration of hydrocortisone (12 to 15 mg/m2 per day), which varied depending on patient age (3 × 5 mg/d for CAH21 newborns, rapidly decreasing to 12 to 15 mg/m2 per day), whether they had reached puberty (15 to 17.5 mg/m2 per day), and their intercurrent diseases. Mineralocorticoid treatment consisted of the administration of 50 µg of 9α fludrocortisone per day, on average. Patient treatment was regularly adapted during clinical, biological, and hormonal follow-up. Adherence was estimated through clinical monitoring of growth rate, bone age, and the measurement of serum adrenocorticotropic hormone and renin.
The patient with a 17-hydroxylase enzyme anomaly, characterized by a moderate deficiency (composite heterozygote), was an 18-month-old boy. He received three androgenic treatments during the first year. The cortisol assay was performed during different evaluations and was in the basal normal range (91.9, 41.4, and 99.6 ng/mL). Hydrocortisone substitution was then necessary only in case of intercurrent diseases, stress, or surgery.
The patient with a 3β-hydroxysteroid dehydrogenase type 2 deficiency was a 10-year-old girl starting puberty (Prader stage II), treated with hydrocortisone (12.5 mg/m2 per day), 9α-fludrocortisone (50 µg/d), and cyproterone acetate (12.5 mg/d).
All samples were collected in the morning between 8:30 and 10:30 am (fasting state), before treatment. Blood samples were randomly collected during the menstrual cycle for pubertal girls (CAH21, n = 3; non-CAH, n = 2).
C. Statistical Analysis
Quantitative variables are expressed as medians and ranges as specified. We used the Spearman rank test for correlations and the Mann–Whitney or Kruskal–Wallis test for comparisons (GraphPad Prism Software, La Jolla, CA). A P value of <0.05 was considered to indicate a statistically significant difference.
3. Results
Serum concentrations of the 16 steroids are shown in Table 4 for the four groups of patients. The relevant correlations cited in the text are shown in Supplemental Table 4 (94KB, docx) .
Table 4.
Serum Levels of 17OHP, 21DF, P, 21DB, DOC, B, Aldo, A, T, 11βOHA, DHEA, 17OHPreg, Preg, F, E, F/E Ratios, and 11DF in Preterm Newborns (Birth at <37 Weeks of Amenorrhea), Full-Term Newborns, Children >10 Days Up to Young Adult 18 Years Old (Non-CAH), and Children with CAH21
| Variable | Preterm Newborns | Full-Term Newborns | Non-CAH | CAH21 | ANOVA P Value (Kruskal–Wallis) |
|---|---|---|---|---|---|
| 17OHP (ng/mL) | 2.27 (1.27–3.80) | 1.09 (0.14–2.83)a | 0.28 (0.054–2.75) | 11.51 (0.06–299)a | <0.0001 |
| 21DF (ng/mL) | 0.053 (0.027–0.209) | 0.030 (0.027–0.113)a | 0.030 (0.027–0.103)b | 0.980 (0.027–35.17)a | <0.0001 |
| P (ng/mL) male | 0.42 (0.19–1.97) | 0.37 (0.17–1.46)c | 0.07 (0.05–0.40) | 0.28 (0.05–10.14 )a | <0.0001 |
| P (ng/mL) female | 0.52 (0.20–1.29 ) | 0.21 (0.17–0.85)d | 0.07 (0.05–14.37) | 0.29 (0.05–12.58)a | <0.0001 |
| 21DB (ng/mL) | <0.01 (<0.01) | <0.01 (<0.01)c | <0.01 (<0.01) | 0.080 (0.012–3.37)a | <0.0001 |
| DOC (ng/mL) | 0.035 (0.01–0.159) | 0.027 (0.012–0.095)c | 0.036 (0.01–0.270) | 0.017 (0.012–0.290)e | NS |
| B (ng/mL) | 3.67 (0.60–16.70) | 2.18 (0.05–15.55)c | 1.47 (0.05–10.70) | 0.32 (0.05–4.65)a | <0.0001 |
| Aldo (ng/mL) | 0.350 (0.163–1.940) | 0.685 (0.027–2.870)c | 0.112 (0.027–0.645) | 0.055 (0.027–0.278)d | <0.0001 |
| A (ng/mL) male | 0.45 (0.32–0.68) | 0.45 (0.11–1.43)c | 0.33 (0.02–1.35) | 0.93 (0.02–16.54)e | <0.001 |
| A (ng/mL) female | 0.70 (0.35–1.58) | 0.43 (0.08–1.90)c | 0.28 (0.04–2.16) | 0.44 (0.03–9.51)c | NS |
| T (ng/mL) male | 0.44 (0.18–0.73) | 0.18 (0.10–0.95)c | 0.60 (0.02–9.32) | 0.29 (0.03–6.26)c | NS |
| T (ng/mL) female | 0.07 (0.03–0.20) | 0.04 (0.03–0.24)c | 0.07 (0.01–0.48) | 0.10 (0.01–4.14)c | NS |
| 11βOHA (ng/mL) | 1.17 (0.38–2.93) | 0.84 (0.20–2.27)c | 0.73 (0.18–1.54) | 1.05 (0.05–18.91)e | <0.01 |
| DHEA (ng/mL) | 19.93 (6.33–33.08) | 6.87 (0.91–47.0)e | 3.14 (0.43–9.46) | 2.26 (0.22–14.29)c | <0.0001 |
| 17OHPreg (ng/mL) | 20.74 (3.77–35.62) | 7.19 (0.43–41.0)e | 1.58 (0.41–4.80) | 1.32 (0.41–13.39)c | <0.0001 |
| Preg (ng/mL) | 13.07 (0.25–23.37) | 5.80 (3.02–56.22)d | 1.46 (0.20–5.27) | 2.32 (0.20–13.05)c | <0.0001 |
| F (ng/mL) | 62.29 (11.62–116.14) | 20.70 (6.37–103.30)c | 103.10 (0.56–207.10) | 9.87 (0.17–278.90)a | <0.0001 |
| E (ng/mL) | 34.04 (16.89–52.68) | 37.71 (10.50–66.40)c | 22.90 (0.19–54.05) | 3.69 (0.10–53.95)a | <0.0001 |
| F/E | 1.72 (0.45–3.36) | 1.07 (0.22–3.27)d | 3.77 (1.24–7.76) | 3.02 (0.49–5.40)e | <0.0001 |
| 11DF (ng/mL) | 1.26 (0.75–2.88) | 1.23 (0.36–2.35)c | 0.27 (0.05–1.74) | 0.14 (0.05–1.10)e | <0.0001 |
Unless otherwise noted, values are expressed as the median (range). To convert ng/mL to nmol/L, multiply by 3.03 for 17OHP, 21DB, and DOC; 3.18 for P; 2.89 for 21DF, B, and 11DF; 2.77 for Aldo; 3.49 for A; 3.47 for T and DHEA; 3.31 for 11βOHA; 3.01 for 17OHPreg; 3.16 for Preg; 2.76 for F; and 2.78 for E.
Abbreviations: ANOVA, one-way analysis of variance.
P < 0.0001.
Two patients heterozygous for 21 hydroxylase deficiency were excluded (see Discussion).
Nonsignificant (preterm newborns vs term newborns or Non-CAH vs CAH21).
P < 0.05 (preterm newborns vs term newborns or Non-CAH vs CAH21).
P < 0.01 (preterm newborns vs term newborns or Non-CAH vs CAH21).
P < 0.001 (preterm newborns vs term newborns or Non-CAH vs CAH21).
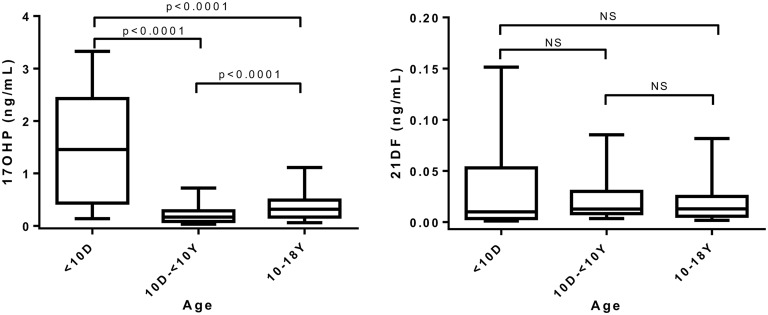
A. 17OHP and 21DF
The 17OHP levels assayed by LC-MS/MS ranged from 1.27 to 3.80 ng/mL in preterm newborns and 0.14 to 2.83 ng/mL in full-term newborns (<10 days), respectively. These levels were significantly higher (P < 0.0001) than those found in older children (Fig. 2). The 21DF concentrations ranged from 0.027 to 0.209 ng/mL in preterm newborns and 0.027 to 0.113 ng/mL in full-term newborns (Table 4). The 17OHP (0.054 to 2.75 ng/mL) in the group of 149 non-CAH patients, which was essentially of adrenal origin before puberty, increased significantly (P < 0.0001) from 10 years of age, whereas 21DF levels remained low (except for two particular cases with much higher 21DF levels: 0.71 and 0.92 ng/mL), with no significant difference between children younger than 10 and children 10 years of age or older (P = not significant) (Fig. 2). Steroid profiles established in 48 treated or untreated cases of CAH-21 showed that basal 17OHP and 21DF levels varied from low concentrations to extremely elevated values, reaching 299 ng/mL for 17OHP and 35 ng/mL for 21DF (Fig. 3; Table 4).
Figure 2.
Box-and-whisker plots (medians, quartiles, and fifth and 95th percentiles) of 17OHP and 21DF serum concentrations and comparisons according to patient age: preterm and full-tem newborns (<10 days old, n = 30), non-CAH children (10 days to 9 years old; n = 40); and non-CAH participants (10 to 18 years old, n = 107). NS, not significant.
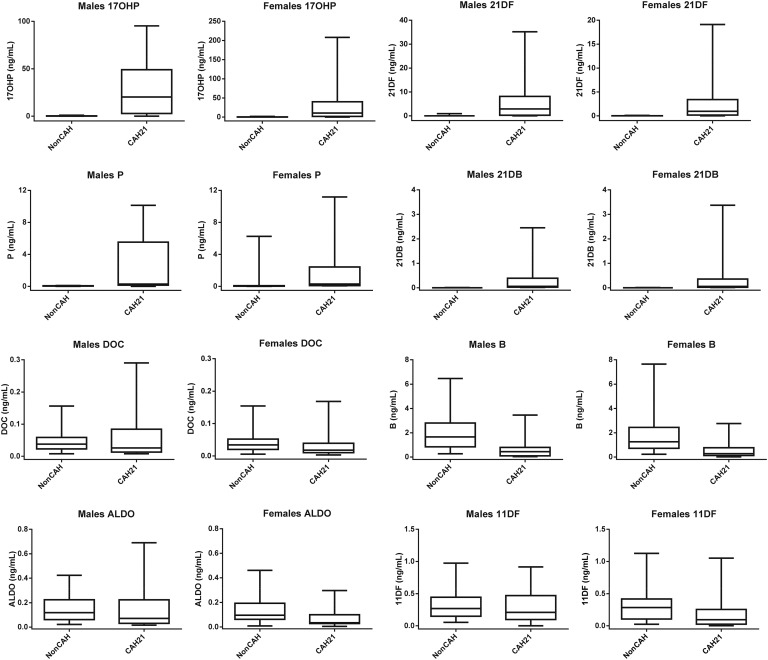
Figure 3.
Box-and-whisker plots (medians, quartiles, and fifth and 95th percentiles) of 17OHP, 21DF, P, 21DB, DOC, B, Aldo, and 11DF in male and female non-CAH21 participants and CAH21 patients.
B. P and 21DB (Also Called 11βOH Progesterone)
In CAH21, serum progesterone concentrations in boys reached high levels, similar to female luteal values (up to 10.14 ng/mL in our patients). These concentrations were significantly higher than those in non-CAH boys (P < 0.0001) (Fig. 3; Table 4).
All non-CAH patients had 21DB levels below the LLOQ (0.012 ng/mL). CAH-21 patients had a wide range of 21DB concentrations (0.012 to 3.37 ng/mL) depending on the CAH treatment.
C. DOC, B, and Aldo
Serum DOC levels in patients with CAH21 were significantly lower (P < 0.0001) that those in non-CAH patients (Fig. 3; Table 4). The B levels showed large variations in all groups and were significantly higher (P < 0.0001) in preterm and full-term newborns than the non-CAH patients. There was a significant correlation between DOC and B levels (rs = 0.675; P < 0.00001) in the non-CAH patients. The CAH21 patients had B levels significantly lower than those of non-CAH patients (P < 0.001). Aldo concentrations in the preterm and full-term newborns were significantly higher (P < 0.0001) than those in non-CAH and CAH21 children. We identified one patient with high blood pressure who exhibited a steroid profile that confirmed a genetically proven 17 hydroxylase deficiency (high corticosterone of 90.4 ng/mL; DOC level, 2.37 ng/mL) (Table 5).
Table 5.
Serum levels (ng/mL) of Aldo, E, F, 11βOHA, 21DF, B, 11DF, A, DOC, 21DB, T, 17OHP, DHEA, 17OHPreg, P, and Preg in a Patient with 17-Hydroxylase Deficiency and a Patient with a 3β-Hydroxysteroid Dehydrogenase Deficiency
| Patient | Aldo | E | F | 11βOHA | 21DF | B | 11DF | A | DOC | 21DB | T | 17OHP | DHEA | 17OHPreg | P | Preg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-Hydroxylase deficiency | 0.11 | 6.82 | 41.4a | 0.03 | 0.07 | 90.4 | 0.59 | 0.01 | 2.37 | 0.14 | 0.01 | 0.36 | <0.13 | 1.17 | 1.46 | 13.0 |
| 3β-Hydroxysteroid dehydrogenase deficiency | 0.03 | 0.81 | 1.93 | 0.06 | 0.03 | 0.02 | 0.26 | 0.06 | 0.01 | 0.01 | 0.25 | 1.35 | 49.1 | 83.1 | 0.06 | 10.8 |
Note the high serum levels of corticosterone, DOC, and 21DB (in bold) in the patient with 17-hydroxylase deficiency and the high serum levels of DHEA, 17-OHPreg (in bold), and Preg with low serum levels of androgens, mineralocorticoids, and glucocorticoids in the patient with a 3β-hydroxysteroid dehydrogenase deficiency.
The cortisol assay was performed at different evaluations and was in the normal basal range (91.9 and 99.6 ng/mL).
D. A, T, and 11βOHA
Androgen levels varied widely in preterm newborns: 0.32 to 1.58 ng/mL for A and 0.03 to 0.73 ng/mL for T. However, T levels were significantly higher in males than females (Table 4). The concentrations of A and 11βOHA were not statistically different between males (n = 12) and females (n = 8) in full-term newborns. However, T levels were significantly higher (P < 0.001) in males (0.10 to 0.95 ng/mL) than in females (0.03 to 0.24 ng/mL).
The data from the non-CAH group, consisting of patients of various ages with various diseases, were used to correlate RIA and LC-MS/MS values (Fig. 1). A levels in males and females were not significantly different, whereas T levels were significantly higher in males than females (P < 0.0001).
The wide range of concentrations of A (0.034 to 16.54 ng/mL), T (0.015 t 6.26 ng/mL), and 11βOHA (0.04 to 18.91 ng/mL) in CAH21 patients are likely explained by glucocorticoid treatments, sex, and age. We found high correlations between A and T (rs = 0.891; P < 0.0001), 11βOHA and A (rs = 0.993; P < 0.00001), and 11βOHA and 21DF (rs = 0.66; P < 0.0001) levels.
E. DHEA, 17OHPreg, and Preg (Δ5 Steroids)
The serum levels of Δ5 steroids in preterm newborns were high, from 6.33 to 33.08 ng/mL for DHEA, 3.77 to 35.62 ng/mL for 17OHpreg, and 0.25 to 23.27 ng/mL for Preg (Table 4). Median serum levels of Δ5 steroids in full-term newborns were lower than in preterm newborns but also varied widely.
The serum levels of Δ5-steroids also varied widely in non-CAH patients but did not surpass 10 ng/mL. The highest levels were 9.5, 4.8, and 5.27 ng/mL for DHEA, 17OHPreg, and Preg, respectively. We found high correlations between DHEA and Preg (rs = 0.908; P < 0.001) and between DHEA and 17OHPreg (rs = 0.918; P < 0.0001) in these patients.
We observed a wide range in the concentrations for each Δ5 steroid in CAH21 patients (0.22 to 14.29 ng/mL for DHEA, 0.41 to 13.39 ng/mL for 17OHPreg, and 0.2 to 13.05 ng/mL for Preg) because of the glucocorticoid treatments.
Here, we also report a typical serum steroid profile of a patient with genetically proven 3β-hydroxysteroid dehydrogenase adrenal deficiency who had high serum DHEA and 17OHPreg levels and low serum levels of cortisol, androgens, and mineralocorticoids (Table 5).
F. E and F
The median F/E ratios in preterm and full-term newborns were 1.72 and 1.07, respectively (Table 4). However, the F levels in a few serum samples of preterm and full-term newborns were lower than E levels. The F/E ratios were between 1.24 and 7.76 in non-CAH patients, and the F and E levels were significantly correlated (r = 0.650; P < 0.0001). In CAH21 patients, the F and E levels depended on the treatment, and the highest F/E ratio measured was 5.4.
G. 11DF
The maximum 11DF levels in preterm and full-term newborns were 2.88 ng/mL and 2.35 ng/mL, respectively (Fig. 3; Table 4). The highest 11DF levels in non-CAH and CAH21 patients were 1.74 ng/mL and 1.1 ng/mL, respectively.
4. Discussion
Levels of 17OHP measured by RIA in preterm and full-term newborns are higher than when measured by LC-MS/MS and could lead to misdiagnosis or uncertainty. LC-MS/MS provides better diagnostic confidence, especially during the neonatal period. Available 17OHP values obtained by RIA in preterm (n = 14 samples from 10 babies) and full-term (n = 11 samples from 20 babies) newborns were high, resulting in false-positive results for six of the 21 neonates (Table 3); this was not the case for the LC-MS/MS values. The artifactually high 17OHP levels may be related to cross-reactions with 3β-hydroxy-5-ene steroids [12], such as the high levels of DHEA and 17OHPreg that we reported (Tables 3 and 4). Nevertheless, 17OHP serum levels measured in preterm and full-term newborns by LC-MS/MS were higher than those measured in prepubertal children (Fig. 2) and may be related to reduced 11β-hydroxylase activity in preterm newborns, as previously reported [13].
21DF production in healthy persons is negligible but increases markedly in patients with CAH21. Two decades ago, we and then other researchers reported the usefulness of 21DF measurement by RIA [14–17]; these studies were followed by others using LC-MS/MS [18–22] (Table 6). The development of MS now permits the measurement of 21DF levels by LC-MS/MS, simultaneously with other steroids of interest, by using a small volume of serum. The upper basal level of 21DF (Table 6) that we detected by LC-MS/MS (0.209 ng/mL) was similar to that previously found by RIA (0.15 ng/mL) by our group in a pediatric population without CAH [16]. The highest basal level of 21DF serum measured by RIA or LC-MS/MS (Table 6) reported by several authors (>0.4 ng/mL) was based on studies that included controls that were likely heterozygous carriers of CAH-21 [16, 23]. The low 21DF levels observed in preterm and full-term newborns has made 21DF a useful additional marker to exclude CAH in these patients. Moreover, 21DF can provide a differential diagnosis between CAH with 21 hydroxylase deficiency and normal preterm newborns by blood spot analysis, whereas 17OHP cannot [24].
Table 6.
Chronology of Published Reports of 21 Deoxycortisol and 21 Deoxycorticosterone: Upper Basal Serum Values in Non-CAH
| Study, Year [Reference] | Methods | Studied Populations | Upper Levels or Ranges (ng/mL) |
|---|---|---|---|
| 21DF | |||
| Milewicz et al., 1984 [14] | RIA | Men (mean + 2 SDs) | 0.152 |
| Children <5 d (mean + 2 SDs) | 0.180 | ||
| Gueux et al., 1985 [15] | RIA | Men (mean + 2 SDs) | 0.190 |
| Fiet et al., 1988 [16] | RIA | Men: upper value | 0.150 |
| Women: follicular phase | 0.300 | ||
| Women: luteal phase | 0.260 | ||
| Prepubertal children: upper value | 0.150 | ||
| Fiet et al., 1994 [2] | RIA | Women: mean + 2 SDs | 0.170 |
| Tonetto-Fernandes et al., 2006 [17] | RIA | Normal children <15 y | 0.280–0.430 |
| Carvalho et al., 2008 [18] | LC-MS/MS | Adult controls | 0.380 |
| Costa-Barbosa et al., 2010 [19] | LC-MS/MS | Controls | 0.240 |
| Kulle et al., 2015 [20] | LC-MS/MS | Healthy volunteers | 0.030–0.140 |
| Turcu et al., 2015 [21] | LC-MS/MS | Controls | 0.22–0.685 |
| Travers et al., 2016 [22] | LC-MS/MS | Controls | 0.05–0.25 |
| Current study | LC-MS/MS | Preterms | 0.023–0.209 |
| Newborns <10 d | 0.030–0.113 | ||
| Children <18 y | 0.030–0.109 | ||
| 21DB | |||
| Gueux et al., 1987 [25] | RIA | Men (mean + 2 SDs) | 0.008 |
| Women (mean + 2 SDs) | 0.024 | ||
| Fiet et al., 1989 [26] | RIA | Men | 0.014 |
| Women, follicular phase | 0.013 | ||
| Women: luteal phase | 0.018 | ||
| Prepubertal: highest value | 0.015 | ||
| Turcu et al., 2015 [21] | LC-MS/MS | Controls | 0–0 |
| Current study | LC-MS/MS | Preterm and full-term newborns, children <18 y | <0.012 |
SD, standard deviation.
In the current study, we detected very stable and low basal levels of 21DF (<0.103 ng/mL) in all but two of the 149 non-CAH children; these two patients had levels of 0.70 ng/mL and 0.91 ng/mL, likely corresponding to heterozygous carriers of CAH21 [16, 23]. These stable 21DF levels are in contrast to 17OHP levels, which depend on adrenal and gonadal secretions and consequently increase at puberty (Figs. 2 and 3).
In the 48 cases of CAH21, serum 17OHP and 21DF concentrations were 100 to 1000 times the levels of these steroids in non-CAH children. The levels of these two steroids were closely correlated (rs = 0.949; P < 0.0001).
In CAH21 patients, P serum levels correlated with those of 17OHP (rs = 0.890; P < 0.00001), and several male patients in this group exhibited P serum levels as high as those in the luteal phase in women. P serum levels thus appear to be good additive marker for 21-hydroxylase deficiency (Fig. 3).
Our group previously postulated the possible direct 11β hydroxylation of P in 21 hydroxylase deficiency to form 21DB (also called 11β hydroxy progesterone) and quantified this steroid for the first time by RIA [25, 26] (Table 6). This allowed us to show an increase in the serum concentration of this steroid in classic and nonclassic CAH21 [25, 26]. In the current study, we found very low levels of 21DB in non-CAH patients by LC-MS/MS, often below the LLOQ (0.012 ng/mL). This is consistent with the 21DB levels we found previously by using RIA [26] and is in agreement with recent results obtained by using LC-MS/MS [21]. However, the 21DB serum levels in CAH21 patients correlated with those of 21DF (rs = 0.661; P < 0.0001) and P (rs = 0.663; P < 0.0001), and we found 21DB levels of up to 3.37 ng/mL. 21BD serum levels varied widely, depending on treatment.
Overall concentrations of mineralocorticoids (DOC, B, Aldo) were lower in CAH21 than non-CAH patients (Fig. 3; Table 4), as previously reported [20, 21], likely due to 21 hydroxylase deficiency in the mineralocorticoid pathway or fludrocortisone treatment. The high Aldo serum levels found in normal preterm and full-term newborns may be related to physiologic partial aldosterone resistance during the neonatal period [27].
Here, we measured the concentrations of 16 steroids in genetically proven 17 hydroxylase deficiency with very high levels of DOC and B, confirming the previously established diagnosis (Table 5). We also showed elevated levels of 21DB, which has not been previously reported.
The A and T levels varied widely, with significantly higher T levels in male than female full-term newborns [28]. The levels of 11βOHA, a rarely assayed androgen that originates mainly from 11β-hydroxylation of A [29], showed a very close correlation with levels of A (rs = 0.993). Given that 11βOHA is also produced from cortisol [30], we suggest that 21DF may also contribute to the formation of this 11β-hydroxylated androgen in CAH21.
Unlike A and T, which come from adrenals and gonads, 11βOHA is strictly secreted by the adrenals. It is inactive on androgen receptors but reflects the adrenal contribution to circulating androgens in CAH21. We found a very wide range of 11βOHA values in CAH21 (0.025 to 18.91 ng/mL), and the median level (1.05 ng/mL) was below that previously reported by using RIA (3.4 ng/mL) [2] or LC-MS/MS [31]. This can be explained by the treatment of patients.
The high serum levels of DHEA, 17OHPreg, and Preg in preterm and full-term newborns, as previously reported [32], may be due to the persistence of the adrenal inner fetal zone after birth [33, 34]. The values of these steroids in non-CAH children were similar to those previously reported [35].
The typical basal serum steroid profile of a genetically proven 3β-hydroxysteroid dehydrogenase type 2 adrenal deficiency patient, with high levels of Δ5 steroids and low levels of the other reported steroids (Table 5), agrees with previously published data [36]. The near-normal androgen levels can be explained by suppression of pituitary gland function by cyproterone acetate treatment.
The high levels of E [37, 38] in preterm and full-term newborns reflect the persistence of the fetal zone for several weeks after birth, with high expression of 11β-hydroxy steroid dehydrogenase type 2 [39]. The median F/E ratio in non-CAH children (3.77) was similar to that found by others (4.11) in healthy persons [40]. The determination of E and F serum levels and the F/E ratio is challenging because a high F/E ratio in the context of high blood pressure, hypokalemia, or hypoaldosteronemia leads to the suspicion of genetic 11β-hydroxy steroid dehydrogenase type 2 deficiency [41]. In CAH21, the E and F levels depend on the treatment, and the highest F/E ratio was 5.35.
The 11DF levels were significantly higher in preterm and full-term newborns than prepubertal non-CAH patients (P < 0.0001), and, as previously discussed for 17OHP, these higher 11DF levels may be related to low physiologic 11β-hydroxylase activity, as previously described for preterm babies [13].
The highest level of 11DF was 1.74 ng/mL in non-CAH children and 1.1 ng/mL in CAH-21 children (Table 4). Knowledge of these limits is important for the detection of cases of 11β-hydroxylase deficiency, which we did not encounter in this study.
Our study had some limitations, notably the various treatments received by many patients. The strength of our work, however, has been its ability to investigate a large number of steroids of adrenal, gonadal, or mixed origin that are useful for the diagnosis and monitoring of the various types of CAH, particularly in pediatrics, using a small volume of serum (150 µL), under real-life conditions. This approach could be suitable for routine medical analysis. We also showed the comparative advantage of LC-MS/MS over RIA for avoiding false-positive results in the diagnosis of CAH21 in neonatal patients.
Finally, we demonstrated the stable and low serum levels of 21DF (upper levels, about 0.2 ng/mL) and undetectable serum levels of 21DB in non-CAH patients. These two 21 deoxysteroids of strictly adrenal origin specifically increase in CAH21 patients. The 21DF assay provides important information on the glucocorticoid pathway, whereas the 21DB assay explores the enzymatic block in the mineralocorticoid pathway.
Acknowledgments
The authors thank the following participating physicians: Professor I. Netchine, Dr. F. Brioude, Dr. M. Houang, and Dr. B. Esteva, for referring patients and collecting clinical data. They also thanks Alex Edelman and William Hempel & Associates for editing the manuscript.
Acknowledgments
This work was supported by the APHP, Hôpitaux Universitaires Est Parisien-Paris, the Université Pierre et Marie Curie (Sorbonne Universités UPMC-Paris 6), the Institut National de la Santé et de la Recherche Médicale (INSERM), and the Agence Française de lutte contre le Dopage (AFLD).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 11βOHA
- 11β hydroxy Δ4-androstenedione
- 11DF
- 11 deoxycortisol
- 17OHP
- 17OH progesterone
- 17OHPreg
- 17OH pregnenolone
- 21DB
- 21 deoxycorticosterone
- 21DF
- 21 deoxycortisol
- A
- Δ4-androstenedione
- Aldo
- aldosterone
- B
- corticosterone
- CAH
- congenital adrenal hyperplasia
- CAH21
- CAH due to steroid 21-hydroxylase deficiency
- DHEA
- dehydroepiandrosterone
- DOC
- 11 deoxycorticosterone
- E
- cortisone
- F
- cortisol
- LC-MS/MS
- liquid chromatography/tandem mass spectrometry
- LLOQ
- lower limit of quantitation
- P
- progesterone
- Preg
- pregnenolone
- QC
- quality control
- RIA
- radioimmunoassay
- T
- testosterone.
References and Notes
- 1.Koal T, Schmiederer D, Pham-Tuan H, Röhring C, Rauh M. Standardized LC-MS/MS based steroid hormone profile-analysis. J Steroid Biochem Mol Biol. 2012;129(3-5):129–138. [DOI] [PubMed] [Google Scholar]
- 2.Fiet J, Gosling JP, Soliman H, Galons H, Boudou P, Aubin P, Belanger A, Villette JM, Julien R, Brérault JL, Burthier JM, Morineau G, Al Halnak A, Vexiau P. Hirsutism and acne in women: coordinated radioimmunoassays for eight relevant plasma steroids. Clin Chem. 1994;40(12):2296–2305. [PubMed] [Google Scholar]
- 3.Jiang H, Cao H, Zhang Y, Fast DM. Systematic evaluation of supported liquid extraction in reducing matrix effect and improving extraction efficiency in LC-MS/MS based bioanalysis for 10 model pharmaceutical compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;891-892:71–80. [DOI] [PubMed] [Google Scholar]
- 4.Owen LJ, Keevil BG. Supported liquid extraction as an alternative to solid phase extraction for LC-MS/MS aldosterone analysis? Ann Clin Biochem. 2013;50(Pt 5):489–491. [DOI] [PubMed] [Google Scholar]
- 5.Owen LJ, Wu FC, Keevil BG. A rapid direct assay for the routine measurement of oestradiol and oestrone by liquid chromatography tandem mass spectrometry. Ann Clin Biochem. 2014;51(Pt 3):360–367. [DOI] [PubMed] [Google Scholar]
- 6.Meunier C, Blondelle D, Faure P, Baguet JP, Le Goff C, Chabre O, Ducros V. Development and validation of a method using supported liquid extraction for aldosterone determination in human plasma by LC-MS/MS. Clin Chim Acta. 2015;447:8–15. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Gugten JG, Crawford M, Grant RP, Holmes DT. Supported liquid extraction offers improved sample preparation for aldosterone analysis by liquid chromatography tandem mass spectrometry. J Clin Pathol. 2012;65(11):1045–1048. [DOI] [PubMed] [Google Scholar]
- 8.Kruve A, Rebane R, Kipper K, Oldekop ML, Evard H, Herodes K, Ravio P, Leito I. Tutorial review on validation of liquid chromatography-mass spectrometry methods: part II. Anal Chim Acta. 2015;870:8–28. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. Guidances (drugs). Bioanalytical method validation, Revision 1. 2013. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/. Accessed 17 Febuary 2016.
- 10.Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Owen WE, Bunker AM, Meikle AW. Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clin Chem. 2006;52(8):1559–1567. [DOI] [PubMed] [Google Scholar]
- 11.Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165(2-3):216–224. [DOI] [PubMed] [Google Scholar]
- 12.Wong T, Shackleton CH, Covey TR, Ellis G. Identification of the steroids in neonatal plasma that interfere with 17 α-hydroxyprogesterone radioimmunoassays. Clin Chem. 1992;38(9):1830–1837. [PubMed] [Google Scholar]
- 13.Kamrath C, Hartmann MF, Boettcher C, Wudy SA. Reduced activity of 11β-hydroxylase accounts for elevated 17α-hydroxyprogesterone in preterms. J Pediatr. 2014;165(2):280–284. [DOI] [PubMed] [Google Scholar]
- 14.Milewicz A, Vecsei P, Korth-Schütz S, Haack D, Rösler A, Lichtwald K, Lewicka S, von Mittelstaedt G. Development of plasma 21-deoxycortisol radioimmunoassay and application to the diagnosis of patients with 21-hydroxylase deficiency. J Steroid Biochem. 1984;21(2):185–191. [DOI] [PubMed] [Google Scholar]
- 15.Gueux B, Fiet J, Pham-Huu-Trung MT, Villette JM, Gourmelen M, Galons H, Brerault JL, Vexiau P, Julien R. Radioimmunoassay for 21-deoxycortisol: clinical applications. Acta Endocrinol (Copenh). 1985;108(4):537–544. [DOI] [PubMed] [Google Scholar]
- 16.Fiet J, Gueux B, Gourmelen M, Kuttenn F, Vexiau P, Couillin P, Pham-Huu-Trung MT, Villette JM, Raux-Demay MC, Galons H, Julien R. Comparison of basal and adrenocorticotropin-stimulated plasma 21-deoxycortisol and 17-hydroxyprogesterone values as biological markers of late-onset adrenal hyperplasia. J Clin Endocrinol Metab. 1988;66(4):659–667. [DOI] [PubMed] [Google Scholar]
- 17.Tonetto-Fernandes V, Lemos-Marini SH, Kuperman H, Ribeiro-Neto LM, Verreschi IT, Kater CE. Serum 21-deoxycortisol, 17-hydroxyprogesterone, and 11-deoxycortisol in classic congenital adrenal hyperplasia: clinical and hormonal correlations and identification of patients with 11beta-hydroxylase deficiency among a large group with alleged 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(6):2179–2184. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho VM, Nakamura OH, Vieira JG. Simultaneous quantitation of seven endogenous C-21 adrenal steroids by liquid chromatography tandem mass spectrometry in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872(1-2):154–161. [DOI] [PubMed] [Google Scholar]
- 19.Costa-Barbosa FA, Tonetto-Fernandes VF, Carvalho VM, Nakamura OH, Moura V, Bachega TA, Vieira JG, Kater CE. Superior discriminating value of ACTH-stimulated serum 21-deoxycortisol in identifying heterozygote carriers for 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2010;73(6):700–706. [DOI] [PubMed] [Google Scholar]
- 20.Kulle AE, Riepe FG, Hedderich J, Sippell WG, Schmitz J, Niermeyer L, Holterhus PM. LC-MS/MS based determination of basal- and ACTH-stimulated plasma concentrations of 11 steroid hormones: implications for detecting heterozygote CYP21A2 mutation carriers. Eur J Endocrinol. 2015;173(4):517–524. [DOI] [PubMed] [Google Scholar]
- 21.Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100(6):2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travers S, Martinerie L, Bouvattier C, Boileau P, Lombès M, Pussard E Multiplexed steroid profiling of gluco- and mineralocorticoids pathways using a liquid chromatography tandem mass spectrometry method. J Steroid Biochem Mol Biol. 2017;165(Pt B):202–211. [DOI] [PubMed] [Google Scholar]
- 23.Gourmelen M, Gueux B, Pham Huu Trung MT, Fiet J, Raux-Demay MC, Girard F. Detection of heterozygous carriers for 21-hydroxylase deficiency by plasma 21-deoxycortisol measurement. Acta Endocrinol (Copenh). 1987;116(4):507–512. [DOI] [PubMed] [Google Scholar]
- 24.Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, Sander J. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2007;92(7):2581–2589. [DOI] [PubMed] [Google Scholar]
- 25.Gueux B, Fiet J, Galons H, Boneté R, Villette JM, Vexiau P, Pham-Huu-Trung MT, Raux-Eurin MC, Gourmelen M, Brérault JL, Julien R, Dreux C. The measurement of 11 beta-hydroxy-4-pregnene-3,20-dione (21-deoxycorticosterone) by radioimmunoassay in human plasma. J Steroid Biochem. 1987;26(1):145–150. [DOI] [PubMed] [Google Scholar]
- 26.Fiet J, Gueux B, Raux-DeMay MC, Kuttenn F, Vexiau P, Brerault JL, Couillin P, Galons H, Villette JM, Julien R, Dreux C. Increased plasma 21-deoxycorticosterone (21-DB) levels in late-onset adrenal 21-hydroxylase deficiency suggest a mild defect of the mineralocorticoid pathway. J Clin Endocrinol Metab. 1989;68(3):542–547. [DOI] [PubMed] [Google Scholar]
- 27.Martinerie L, Pussard E, Foix-L’Hélias L, Petit F, Cosson C, Boileau P, Lombès M. Physiological partial aldosterone resistance in human newborns. Pediatr Res. 2009;66(3):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greaves RF, Pitkin J, Ho CS, Baglin J, Hunt RW, Zacharin MR. Hormone modeling in preterm neonates: establishment of pituitary and steroid hormone reference intervals. J Clin Endocrinol Metab. 2015;100(3):1097–1103. [DOI] [PubMed] [Google Scholar]
- 29.Fiet J, Gourmel B, Villette JM, Brerault JL, Julien R, Cathelineau G, Dreux C. Simultaneous radioimmunoassay of androstenedione, dehydroepiandrosterone and 11-beta-hydroxyandrostenedione in plasma. Horm Res. 1980;13(3):133–149. [DOI] [PubMed] [Google Scholar]
- 30.Axelrod LR, Kraemer DC, Burdett J Jr, Goldzieher JW. Biosynthesis of 11 -hydroxyandrostenedione by human and baboon adrenals. Acta Endocrinol (Copenh). 1973;72(3):545–550. [DOI] [PubMed] [Google Scholar]
- 31.Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riepe FG, Mahler P, Sippell WG, Partsch CJ. Longitudinal study of plasma pregnenolone and 17-hydroxypregnenolone in full-term and preterm neonates at birth and during the early neonatal period. J Clin Endocrinol Metab. 2002;87(9):4301–4306. [DOI] [PubMed] [Google Scholar]
- 33.Nomura S. Immature adrenal steroidogenesis in preterm infants. Early Hum Dev. 1997;49(3):225–233. [DOI] [PubMed] [Google Scholar]
- 34.Shimozawa K, Saisho S, Yata J, Kambegawa A. Age-related changes in serum 17-hydroxypregnenolone and 17-hydroxypregnenolone sulfate concentrations in human infancy and childhood. Endocrinol Jpn. 1988;35(2):189–195. [DOI] [PubMed] [Google Scholar]
- 35.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56(7):1138–1147. [DOI] [PubMed] [Google Scholar]
- 36.Mermejo LM, Elias LL, Marui S, Moreira AC, Mendonca BB, de Castro M. Refining hormonal diagnosis of type II 3beta-hydroxysteroid dehydrogenase deficiency in patients with premature pubarche and hirsutism based on HSD3B2 genotyping. J Clin Endocrinol Metab. 2005;90(3):1287–1293. [DOI] [PubMed] [Google Scholar]
- 37.Martinerie L, Pussard E, Meduri G, Delezoide AL, Boileau P, Lombès M. Lack of renal 11 beta-hydroxysteroid dehydrogenase type 2 at birth, a targeted temporal window for neonatal glucocorticoid action in human and mice. PLoS One. 2012;7(2):e31949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujitaka M, Jinno K, Sakura N, Takata K, Yamasaki T, Inada J, Sakano T, Horino N, Kidani K, Ueda K. Serum concentrations of cortisone and cortisol in premature infants. Metabolism. 1997;46(5):518–521. [DOI] [PubMed] [Google Scholar]
- 39.Fahlbusch FB, Ruebner M, Rascher W, Rauh M. Combined quantification of corticotropin-releasing hormone, cortisol-to-cortisone ratio and progesterone by liquid chromatography-Tandem mass spectrometry in placental tissue. Steroids. 2013;78(9):888–895. [DOI] [PubMed] [Google Scholar]
- 40.Homma M, Tanaka A, Hino K, Takamura H, Hirano T, Oka K, Kanazawa M, Miwa T, Notoya Y, Niitsuma T, Hayashi T. Assessing systemic 11beta-hydroxysteroid dehydrogenase with serum cortisone/cortisol ratios in healthy subjects and patients with diabetes mellitus and chronic renal failure. Metabolism. 2001;50(7):801–804. [DOI] [PubMed] [Google Scholar]
- 41.Morineau G, Boudi A, Barka A, Gourmelen M, Degeilh F, Hardy N, al-Halnak A, Soliman H, Gosling JP, Julien R, Brerault JL, Boudou P, Aubert P, Villette JM, Pruna A, Galons H, Fiet J. Radioimmunoassay of cortisone in serum, urine, and saliva to assess the status of the cortisol-cortisone shuttle. Clin Chem. 1997;43(8 Pt 1):1397–1407. [PubMed] [Google Scholar]