Abstract
Key Messages:
Octreotide can be used as an adjunctive therapy to increase phosphorus levels in patients with tumor-induced osteomalacia. Malignant phosphaturic mesenchymal tumor (PMT) may benefit from treatment with peptide receptor radionucleotide therapy.
Context:
The success of treatment modalities for malignant PMT is limited. Octreotide has been used to treat hypophosphatemia in patients with tumor-induced osteomalacia with equivocal results. To our knowledge, there are no reports of octreotide or peptide receptor radionuclide therapy use for malignant PMT.
Case Description:
We report a 40-year-old man having hypophosphatemia, phosphaturia (tubular maximum of phosphorus corrected for glomerular filtration rate of <2.5 mg/dL), and somatostatin avid lesions in the right foot region with metastasis to both lungs. The patient had been subjected to resection of the primary tumor from the foot with thoracoscopic removal of the lung secondaries. Histology from all three lesions showed a spindle cell soft tissue tumor with a high mitotic index and somatostatin receptor 2 and 5 positivity. A trial of subcutaneous octreotide therapy at a dose of 100 μg thrice daily resulted in an increase in serum phosphorus levels from an average of 1.44 mg/dL to an average of 2.3 mg/dL. Finally, the affected limb was amputated, and the hypophosphatemia persisted postoperatively. In view of persistent hypophosphatemia and transient response to octreotide, the patient was administered four cycles of peptide receptor radionuclide therapy using 177Lutetium, which showed moderate improvement of serum phosphorus levels.
Conclusion:
Although octreotide use has been reported in four patients with benign PMT, to our knowledge, this is the first case of malignant PMT that has used peptide receptor radionuclide therapy in the treatment of malignant PMT. This moderately beneficial evidence is likely to guide the future use of radionuclide treatments in such tumors.
Keywords: malignant tumor induced osteomalacia, metastatic tumor induced osteomalacia, metastatic phosphaturic mesenchymal tumor, octreotide, peptide receptor, radionuclide (PRRT), lutetium
Octreotide was used in osteomalacia caused by a malignant phosphaturic tumor, with moderate results; peptide receptor radionuclide therapy with 177Lutetium then produced a moderate response lasting 6 months.
Tumor-induced osteomalacia (TIO) is an acquired renal phosphate wasting disorder caused by mesenchymal tumors. A case of TIO due to a metastatic phosphaturic mesenchymal tumor (PMT), which is rare, is presented. This case report highlights the moderate response of phosphate levels to octreotide treatment and targeted peptide receptor radionuclide therapy using 177Lutetium-tagged DOTATATE.
1. Case Report
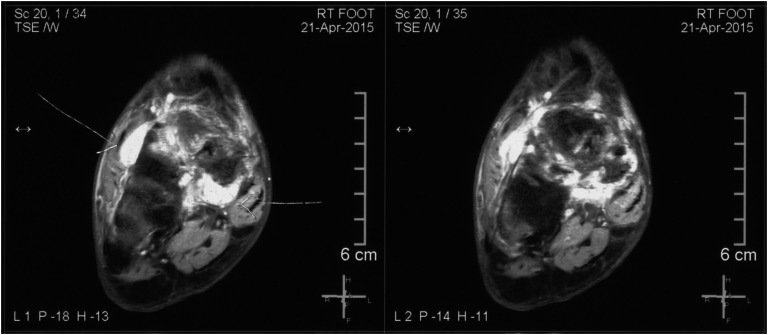
A 40-year-old man was referred to this center with insidious-onset low back pain that he had sustained for 2.5 years, followed by progressive and severe weakness of both lower limbs, both upper limbs, and trunk, in that order. On the neurologist’s evaluation, he was found to have an undisplaced fracture of the right neck of the femur with hypophosphatemia (0.7 to 1.1 mg/dL). TIO was suspected, and somatostatin receptor scintigraphy (99mTc-HYNIC-TOC) showed a somatostatin avid lesion in the right tarsal region that was confirmed by magnetic resonance imaging (Fig. 1). The patient underwent two surgeries of the right tarsal region, and histopathology confirmed PMT. In view of the persistent hypophosphatemia, 6 months later, somatostatin imaging (68 Ga-DOTANOC positron emission tomography/computed tomography) was repeated, which revealed uptake in the left (lingular segment of the upper lobe) as well as right lung (superior segment of the lower lobe) in addition to a residual lesion in the right tarsal region.
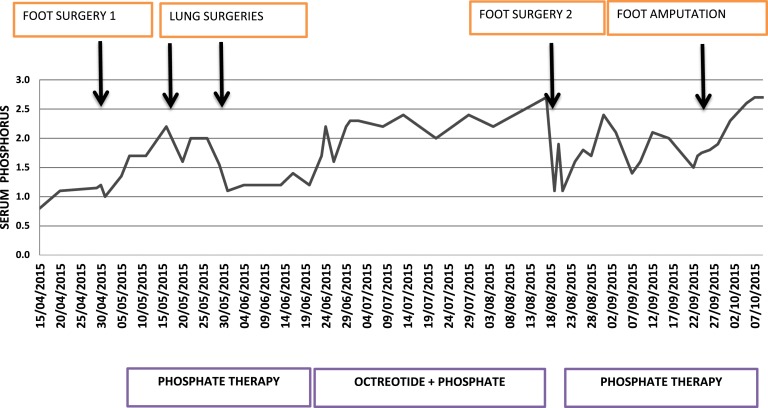
Figure 1.
Serum phosphorus levels and variation with different interventions.
Evaluation at our center revealed serum phosphorus of 0.8 mg/dL, with a tubular maximum of phosphorus corrected for glomerular filtration rate of 0.49 mg/dL. The patient again underwent an unsuccessful attempt at resection of the tumor from the right foot followed by thoracoscopic removal of lung lesions. Histology showed spindle cell soft tissue tumor suggesting a malignant phosphaturic tumor with immunohistochemistry positive for somatostatin receptor subtypes 2 and 5. However, the patient continued to have hypophosphatemia, and a repeat 68 Ga-DOTANOC whole-body positron emission tomography/computed tomography revealed persistent intense uptake in the right foot. In view of somatostatin avidity on imaging and receptor positivity on immunohistochemistry, a trial of short-acting octreotide therapy at a dose of 100 μg thrice daily subcutaneously was given with an improvement in the serum phosphorus level from an average of 1.44 mg/dL to an average of 2.3 mg/dL and a maximum value of 2.7 mg/dL (Fig. 2). This time, we planned to give him targeted 177Lutetium-tagged DOTATATE for four cycles at a 3-month interval because there was improvement in the phosphorus levels after initiation of octreotide therapy, and histopathology was showing somatostatin receptor positivity. Specifically, 177Lutetium-tagged DOTATATE was chosen because of its easier availability in India and properties similar to I-131 and a favorable half-life (6.71 days) and emission properties (both beta and gamma radiations). While waiting for this therapy and in view of the substantial residual foot lesion, the patient opted for below-knee amputation of the right lower limb. A relatively large margin of resection and a better rehabilitation were intended while choosing the below-knee amputation. The first dose of 177Lutetium DOTATATE therapy was then given to tackle the probable micrometastasis that might show up at a later date in the wake of an absence of other effective systemic therapies. Following the 177Luttetium therapy and amputation, there was improvement in serum phosphate levels reaching a maximum of 3.0 mg/dL without any phosphorus replacement, but the improvement was not sustained, and phosphorus levels fell after 2 months, dropping to 1.7 mg/dL. Following the second dose of 177Lutetium therapy, the serum phosphorus levels finally rose to 2.7 mg/dL and persisted for 6 months. Subsequently, the patient had to be started on phosphorus supplementation for maintaining the serum phosphorus levels in the low-normal range. To date, the patient has received four cycles of 177Lutetium therapy. After four cycles of therapy, a 68 Ga-DOTANOC scan was repeated, which revealed no uptake anywhere. Clinically, he has had substantial improvement in bone pain and muscle weakness, and he is ambulant with a right leg prosthesis. The patient is under continued follow-up.
Figure 2.
Primary tumor in right foot seen as T2 hyperintense lesion in the tarsus.
2. Discussion
A. Treatment of Malignant PMTs
Morimoto et al. [1] reported two cases of malignant PMTs. The first one was a 35-year-old woman with papillary thyroid carcinoma with a pelvic bony mass lesion that turned out to be a PMT. The patient underwent transarterial embolization of the pelvic tumor with tumor regrowth after 32 months. Chemotherapy with adriamycin, ifosfamide, gemcitabine, and docetaxel was administered but showed no response, and the patient died of progressive lung metastasis. The second was a patient detected to have hypophosphatemic osteomalacia at the age of 10 years and a pelvic tumor suggestive of benign PMT being found at age 31 years, for which transarterial embolization and later tumor excision were done, resulting in normalization of serum phosphate levels. Two years postsurgery, the patient developed local recurrence with histologically confirmed lung and liver metastasis. Chemotherapy with gemcitabine and docetaxel, adriamycin with ifosfamide, and adriamycin with cisplatin and finally radiation were given, but the patient succumbed.
One case by Sidell et al. [2] was a laryngeal PMT with a high mitotic rate and aggressive regrowth after the first surgery but no metastasis that responded to neoadjuvant chemotherapy, total laryngectomy, and postoperative radiation therapy. The follow-up in the case report is only up to 4 months. Uramoto et al. [3] reported a case of PMT of the tongue, which was resected initially and then at first recurrence. Although the tumor continued to grow, hypophosphatemia improved with radiotherapy. Seijas et al. [4] reported a case where 5 years after documenting hypophosphatemia without a phosphatonin source, the patient presented with a single liver lesion with histologic confirmation as metastatic PMT. Four years later, two pelvic lesions were identified and removed. Again after 2 years, the patient had another recurrence with hepatic metastasis, pulmonary nodules, and rib lesions. Ogose et al. [5] reported a case of pelvic PMT where multiple surgeries were done to remove the primary tumor, and later a hemipelvectomy was required. Each time the surgery helped improve the serum phosphorus levels, but the effect was not sustained. Eventually, the tumor transformed into a high-grade sarcoma. Three more cases of malignant TIO based on histology were reported recently by Sun et al. [6] among a cohort of 40 patients with TIO who had lesions in extremities, but the status of metastasis was not mentioned. Two patients recovered with respect to phosphorus levels with amputation, whereas the third received radiotherapy without benefit.
B. Somatostatin Receptor-Based Therapies for TIO
Previous reports have shown conflicting results of treatment with somatostatin receptor-based therapies [7–10]. In the first case report by Seufert et al. [7] serum phosphorus improved from 1.88 mg/dL to 4.36 mg/dL with octreotide 100 μg thrice daily in addition to the phosphorus supplementation that was being given earlier. However, Paglia et al. [8] observed no benefit in a similar patient. Mékinian et al. [9] showed that long-acting octreotide therapy was not beneficial in a patient with TIO in doses of 10, 20, and 30 mg at an interval of 1 month. Papierska et al. [10] used a long-acting somatostatin analogue in a patient with TIO and found phosphorus levels rising from 1.54 to 2.1 mg/dL within 2 days. Kulshreshtha et al. [11] suggested a limited role of octreotide and cinacalcet in controlling hypophosphatemia over short periods.
To our knowledge, there are no reports of the use of peptide receptor radionuclide therapy in PMT (benign or malignant) and no reports of the use of octreotide in malignant PMT.
3. Conclusion
There is no effective management available for patients with metastatic PMT. In this case report, octreotide at a dose of 300 μg/d subcutaneously has shown improvement in serum phosphorus levels in a patient with malignant PMT for a short period. This case demonstrates that 177Lutetium DOTATATE, used for the treatment of malignant PMT, causes substantial improvement in serum phosphorus levels for at least 6 months, although the long-term impact on serum phosphorous levels, tumor behavior, and patient longevity remains to be seen. Both these interventions could be a ray of hope for this rare and difficult to treat disease.
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- PMT
- phosphaturic mesenchymal tumor
- TIO
- tumor-induced osteomalacia.
References and Notes
- 1.Morimoto T, Takenaka S, Hashimoto N, Araki N, Myoui A, Yoshikawa H. Malignant phosphaturic mesenchymal tumor of the pelvis: a report of two cases. Oncol Lett. 2014;8(1):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidell D, Lai C, Bhuta S, Barnes L, Chhetri DK. Malignant phosphaturic mesenchymal tumor of the larynx. Laryngoscope. 2011;121(9):1860–1863. [DOI] [PubMed] [Google Scholar]
- 3.Uramoto N, Furukawa M, Yoshizaki T. Malignant phosphaturic mesenchymal tumor, mixed connective tissue variant of the tongue. Auris Nasus Larynx. 2009;36(1):104–105. [DOI] [PubMed] [Google Scholar]
- 4.Seijas R, Ares O, Sierra J, Pérez-Dominguez M. Oncogenic osteomalacia: two case reports with surprisingly different outcomes. Arch Orthop Trauma Surg. 2009;129(4):533–539. [DOI] [PubMed] [Google Scholar]
- 5.Ogose A, Hotta T, Emura I, Hatano H, Inoue Y, Umezu H, Endo N. Recurrent malignant variant of phosphaturic mesenchymal tumor with oncogenic osteomalacia. Skeletal Radiol. 2001;30(2):99–103. [DOI] [PubMed] [Google Scholar]
- 6.Sun ZJ, Jin J, Qiu GX, Gao P, Liu Y. Surgical treatment of tumor-induced osteomalacia: a retrospective review of 40 cases with extremity tumors. BMC Musculoskelet Disord. 2015;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seufert J, Ebert K, Müller J, Eulert J, Hendrich C, Werner E, Schuüze N, Schulz G, Kenn W, Richtmann H, Palitzsch KD, Jakob F. Octreotide therapy for tumor-induced osteomalacia. N Engl J Med. 2001;345(26):1883–1888. [DOI] [PubMed] [Google Scholar]
- 8.Paglia F, Dionisi S, Minisola S.. Octreotide for tumor-induced osteomalacia. N Engl J Med. 2002;346(22):1748–1749; author reply 1748–1749. [DOI] [PubMed] [Google Scholar]
- 9.Mékinian A, Ladsous M, Balavoine AS, Carnaille B, Aubert S, Soudan B, Wémeau JL. Curative surgical treatment after inefficient long-acting somatostatin analogues therapy of a tumor-induced osteomalacia. Presse Med. 2011;40(3):309–313. [DOI] [PubMed] [Google Scholar]
- 10.Papierska L, Ćwikła JB, Misiorowski W, Rabijewski M, Sikora K, Wanyura H. FGF23 producing mesenchymal tumor. Case Rep Endocrinol. 2014;2014:492789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulshreshtha B, Dutta D, Dharmshaktu P. Octreotide and cinacalcet have limited role in managing surgically incurable tumor induced osteomalacia. Acta Endocrinol (Bucur). 2015;XI(4):517–523. [Google Scholar]