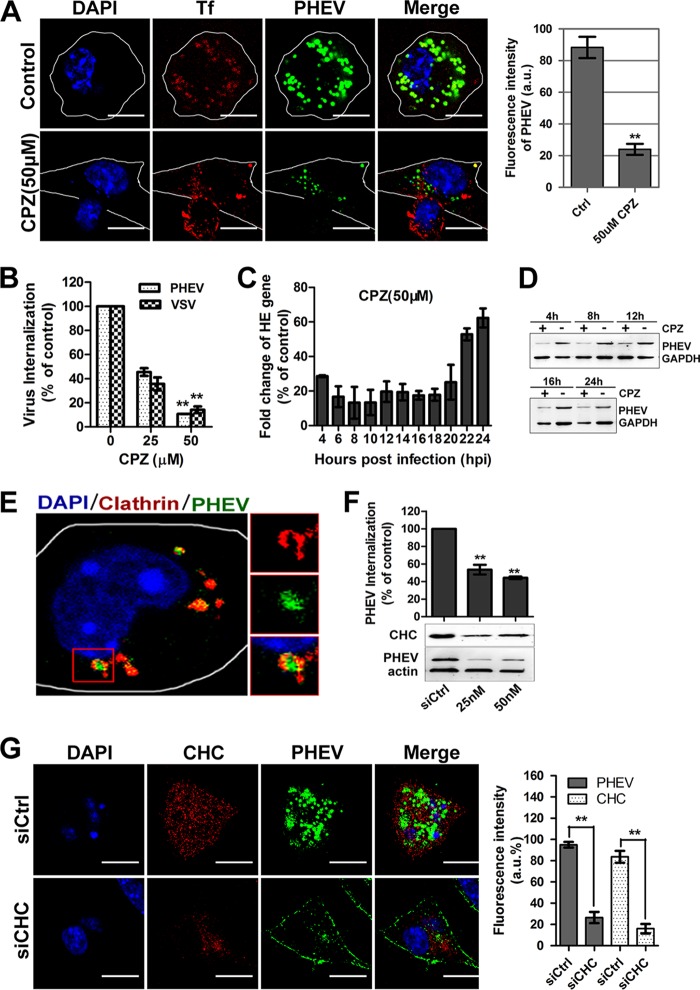
FIG 4.
PHEV infection occurs via a CME-dependent mechanism. (A) The infectivity of PHEV was assessed in Neuro-2a cells that were either mock treated or treated with CPZ (50 μM). The infected cells were fixed and visualized by confocal microscopy. (B) A PHEV internalization assay was carried out with cells pretreated with CPZ at the indicated concentrations. Following internalization, surface-bound viruses were removed and detected by qRT-PCR. The internalization of VSV via CME was used as a control. (C) PHEV RNA replication was inhibited by pretreatment of the cells with CPZ for 1 h, and relative hemagglutinin-esterase (HE) gene expression was assessed using qRT-PCR. (D) PHEV protein synthesis was suppressed by pretreatment with CPZ. Cell lysates were collected, and the expression level of the major viral S protein was measured by Western blotting. (E) PHEV particles colocalized with clathrin. Neuro-2a cells were transfected with DsRed-clathrin prior to incubation with PHEV. The enlarged box indicates PHEV particles (green) that were swallowed into the vesicle structure, which bears clathrin (red) on the outer surface; colocalized signals were widely observed using confocal microscopy. (F) Neuro-2a cells were transfected with an siRNA directed against CHC or a control mock siRNA (siCtrl). Inhibition of PHEV internalization and replication was assessed by qRT-PCR to quantify relative gene expression (top) or by immunoblotting to analyze viral protein synthesis (bottom). The RNAi efficiency against CHC was also examined using an anti-CHC primary antibody. (G) The PHEV load and CHC expression in Neuro-2a cells against a background of CHC depletion were calculated at 24 hpi by confocal microscopy. The relative fluorescence intensities of PHEV and CHC staining are presented in the histogram on the right. Bars, 10 μm. **, P < 0.01.