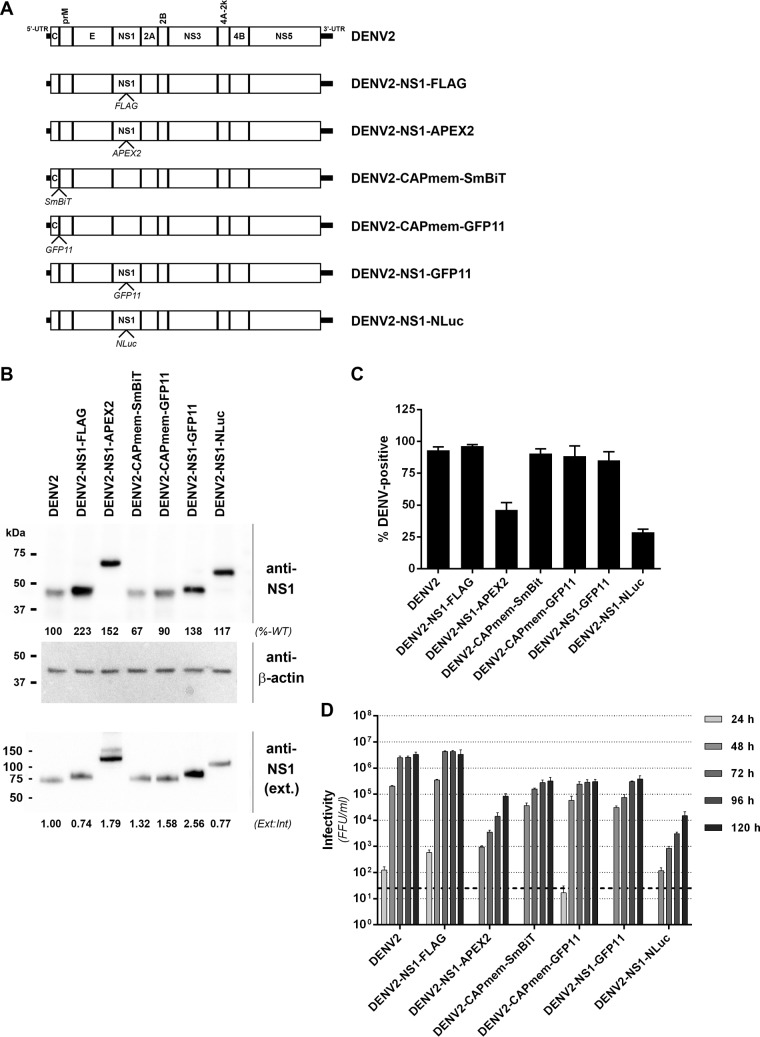
FIG 6.
Characterization of epitope- and reporter-tagged DENV-2 constructs. (A) Guided by the insertional map, a panel of tagged viruses were generated that featured the indicated epitope or reporter protein insertions in capsid, adjacent to the membrane anchor (CAPmem), or NS1, immediately downstream of Lys-174. (B) Huh7.5 cells were electroporated with in vitro-transcribed RNA for the indicated DENV-2 constructs and cultured for 4 days prior to Western blot analysis of NS1 protein. Detection of β-actin served as a loading control. Similarly, supernatants from these cells were cleared by centrifugation and subjected to SDS-PAGE under nonreducing, nondenaturing conditions and Western blotting with anti-NS1 antibody. The numbers below the NS1 Western blots indicate the levels of NS1 protein in whole-cell lysates (normalized to β-actin and expressed as a percentage of wild-type levels [%-WT]; upper panel) and extracellular NS1 protein (expressed as a ratio to intracellular NS1 bands, with the wild-type ratio set to 1 [Ext:Int]; lower panel). (C) Automated immunofluorescence analysis of the proportion of capsid protein-positive Huh-7.5 cells at 4 days postelectroporation with the indicated DENV-2 RNA transcripts. Data are means + the standard deviations (SD; n = 3 for >1,500 cells/electroporation). (D) Infectivity titers were determined by focus forming unit (FFU) assays at 24 to 120 h postelectroporation of Huh-7.5 cells with the indicated DENV-2 RNA transcripts. Data are means + the SD (n = 3). The dashed line indicates the limit of detection of the assay.