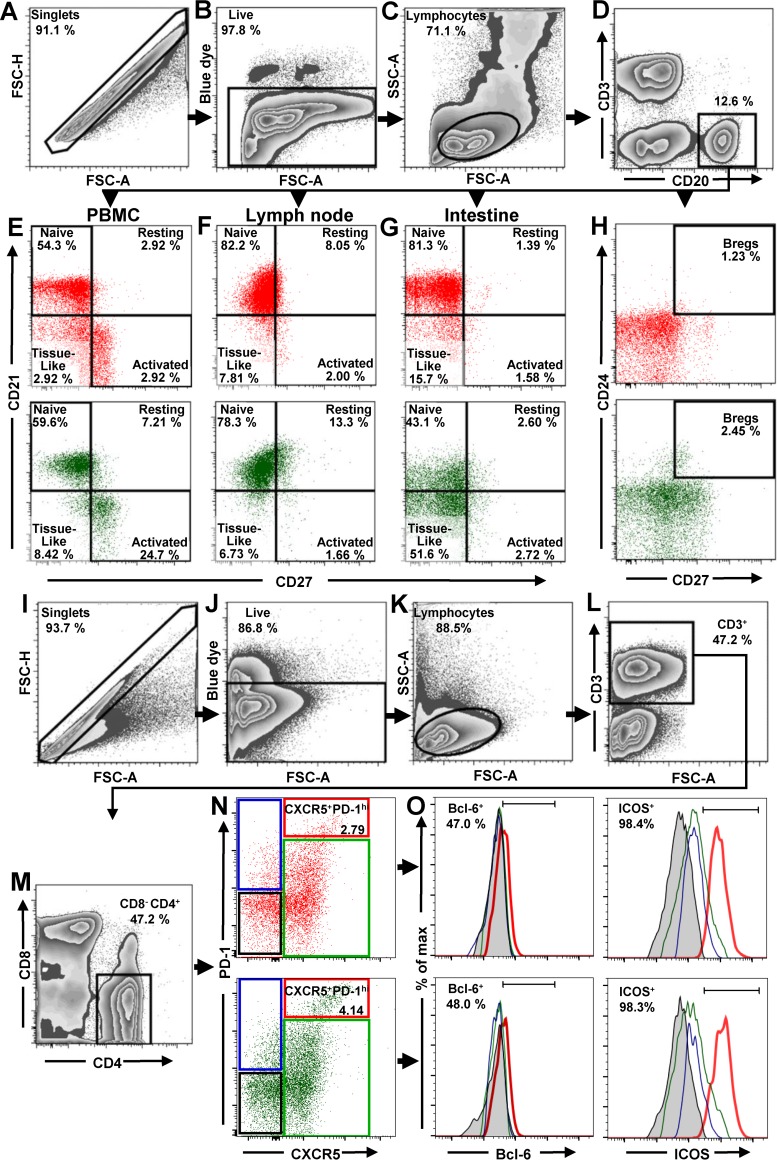
FIG 1.
Flow cytometry strategy for identification of total B cells, B cells subsets, and Tfh cells in PBMCs, LNs, and intestine. The gating strategy to identify total B cells, B cell subsets, and Tfh cells in uninfected AGMs (green dot plots) and uninfected PTMs (red dot plots) is shown. Lymphocytes were gated on singlets, followed by gating on live cells (A to C). B cells from PBMCs, LNs, and intestine were selected by gating on CD20+ (D). CD21 and CD27 markers were used to distinguish naive (CD27neg CD21+), resting memory (CD27+ CD21+), activated memory (CD27+ CD21neg), and tissue-like memory (CD27neg CD21neg) B cells from PBMCs (E), LNs (F), and intestine (G). CD27 and CD24 were used to identify regulatory B cells from PBMCs (H). To characterize Tfh cells in mesenteric and axillary LNs, lymphocytes were gated on singlets, followed by selection of live CD3+ cells (I to L). CD4+ CD8neg cells were then gated (M) and analyzed based on expression of PD-1 and CXCR5. The doubly positive PD-1high CXCR5+ population was used to identify Tfh cells (N). Expression of Bcl-6 and ICOS was assessed in CXCR5neg PD-1neg CD4+ T cells (black square), CXCR5neg PD-1+ CD4+ T cells (blue square), CXCR5+ PD-1neg CD4+ T cells (green square), and CXCR5+ PD-1high CD4+ T cells (red square) (O). FSC-A, forward-scatter area; FSC-H, forward-scatter height; SSC-A, side scatter area.