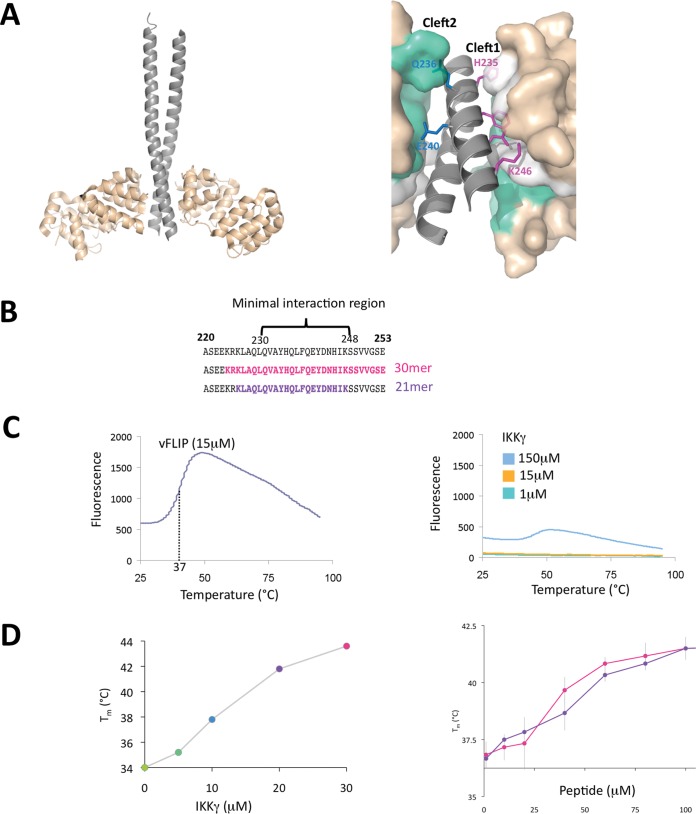
FIG 2.
vFLIP-IKKγ interaction and peptide thermofluor analysis. (A) (Left) Cartoon depiction of the ks-vFLIP–IKKγ heterotetramic complex (PDB code 3CL3). The IKKγ and ks-vFLIP monomers are shown in gray and wheat, respectively. (Right) The ks-vFLIP-IKKγ dimer interface highlighting cleft 1 (white) and cleft 2 (cyan). Residues essential for the formation of the cleft 1 interactions (H235, F238, D242, and K246) are colored magenta, and those that interact with cleft 2 (Q236 and E240) are colored blue. (B) IKKγ ks-vFLIP minimal interacting motif (residues 230 to 248) together with those forming the 30- and 21-mer peptides (pink and violet, respectively) used in the thermofluor studies. (C) Thermal denaturation profiles for ks-vFLIP (left) and IKKγ (right) at various concentrations. The lack of a significant response over the range of concentrations tested for IKKγ is owing to its dimeric coiled configuration that lacks a substantial hydrophobic core. (D) Thermal denaturation of ks-vFLIP in the presence of various concentrations of IKKγ (left) and the 21-mer (pink) and 30-mer (violet) (right) peptides.