Abstract
Podocan, a member of the small leucine-rich repeat proteoglycans (SLRPs), is expressed in vascular endothelial cells with high levels of expression in the sclerotic glomerular lesions of experimental HIV-associated nephropathy. It is also found in vascular smooth muscle cells and is involved in atherosclerosis. Decorin, a protein similar to podocan, also belongs to the SLRP family and is highly expressed in adipose tissues. It is a secreted protein associated with obesity, type 2 diabetes, and diabetic nephropathy. Based on the similarity of podocan to decorin and its functions reported in the renal and cardiovascular systems, we hypothesized that podocan levels might correlate with the occurrence of metabolic syndromes such as obesity, diabetes, and diabetic nephropathy. We found that podocan was highly expressed in the adipose tissue of mice and humans and its expression was regulated by tumor necrosis factor-α in mouse 3T3-L1 adipocytes. In addition, podocan was detected in the plasma, and its levels tended to increase in diet-induced obese C57BL/6J mice and decrease in obese-diabetic KKAy and db/db mice. Podocan messenger RNA (mRNA) levels in the renal cortex correlated negatively with the urinary albumin-to-creatinine ratio, a surrogate marker of glomerular injury in uninephrectomized db/db mice used as a model of diabetic nephropathy. Our results suggest that podocan is involved in kidney function and could be a unique therapeutic target for diabetic nephropathy.
Keywords: podocan, adipose tissue, diabetic nephropathy
We investigated podocan, which was highly expressed in adipose tissue, present in plasma, and associated with diabetic nephropathy in mice.
Podocan, a recently identified member of the small leucine-rich repeat proteoglycans (SLRPs), is produced by podocytes and vascular endothelial cells and binds type 1 collagen [1]. Podocan is a 611-amino-acid-long protein, detected in the sclerotic glomerular lesions of experimental HIV-associated nephropathy [2, 3]. It possesses the predicted signal peptide sequence, MAGSRGLPLLLLVLQLFLGPVLP in mice and MAQSRVLLLLLLLPPQLHL in humans, at its N terminus [4]. Podocan is secreted in the cell culture supernatants of HIV-1 transgenic podocytes and of HEK-293T cells transfected with a mouse podocan expression vector [2]. Podocan modulates the fibrillar structure of collagen in the mature glomerular basement membrane and maintains the integrity of the glomerular filtration barrier [2]. Similar to other SLRPs, podocan shows antimigratory and antiproliferative properties concomitant with changes in p21 and Rho activity [2]. Silencing of the gene encoding podocan significantly promotes cell proliferation via alterations in downstream signaling proteins, including cyclin-dependent kinase 2 and p21 [1]. In addition, podocan is expressed in vascular smooth muscle cells (SMCs) and plays an important role in atherosclerosis [3]. In fact, podocan is strongly and selectively expressed in the arteries of wild-type mice after injury. Podocan-deficient mice show increased arterial lesion formation in response to injury compared with their wild-type littermates [4–6]. In addition, the podocan-deficient mice are characterized by an increase in SMC proliferation, and this effect is exerted through the activation of the Wnt-β-catenin pathway in the SMCs both in vitro and in vivo [6]. Conversely, podocan overexpression in human SMCs inhibits the Wnt-β-catenin pathway and significantly reduces migration and proliferation [6]. A clinical study is currently investigating the predictive factor of circulatory podocan and the Wnt pathway for maladaptive left ventricular response to aortic stenosis [7]. Decorin, which is similar to podocan, also belongs to the SLRP family and is highly expressed in the adipose tissue [8]. It is a secreted protein associated with obesity, type 2 diabetes, and diabetic nephropathy in animals and humans [8–10]. In the cardiac vessels of obese rabbits, the levels of transforming growth factor (TGF)-β messenger RNA (mRNA) and collagen 1 mRNA are increased, whereas that of decorin is significantly reduced compared with those in lean rabbits [11]. In addition, decorin treatment protects the diabetic corpora from apoptosis and fibrosis [12]. Recombinant decorin ameliorates pulmonary structural alterations by downregulating TGF-β and Smad signaling in diabetic rats [13]. Decorin-deficient diabetic mice show aggravated nephropathy due to the overexpression of profibrotic factors, enhanced apoptosis, and mononuclear cell infiltration [9]. They also exhibit advanced glomerular lesions, including diffuse mesangial matrix accumulation and fibrin cap formation [14]. The levels of the SLRPs decorin and biglycan are markedly upregulated in the adipose tissue, particularly in the obese state. According to Bolton et al. [15], they may be involved in the development of obesity and type 2 diabetes by facilitating the expansion of adipose tissue mass. Based on the functions of podocan reported in the renal and cardiovascular systems and information on the role of decorin and other SLRPs in human diseases, we hypothesized that podocan levels might correlate with the occurrence of metabolic syndromes such as obesity, diabetes, and diabetic nephropathy, similar to decorin. Therefore, in this study, we evaluated the association of podocan levels with these diseases.
1. Materials and Methods
A. Chemicals and Reagents
Irbesartan, an angiotensin II receptor blocker, was purchased from LKT Laboratories (St. Paul, MN). Recombinant human TGF-β was purchased from R&D Systems (Minneapolis, MN).
B. Digoxigenin-Labeled Antisense Podocan RNA Probes
The DIG RNA Labeling Kit (Roche Diagnostics, Rotkreuz, Switzerland) was used to prepare two digoxigenin (DIG)-labeled antisense podocan RNA probes. The plasmids pSPT18 [including an open reading frame (ORF) of podocan sequences from Sal1 (at 518 bp from 5′ end) to Sac1 (at 1548 bp from 5′end)] and pSPT18 [including the full-length podocan ORF (approximately 3.2 kbp)] were constructed according to the manufacturer’s protocol [Fig. 1(a)]. Two DIG-labeled antisense podocan RNA probes were then prepared using the T7 RNA polymerase. Probes A and B were approximately 1 kb and 1.8 kb long, owing to the elongation limit of T7 RNA polymerase.
Figure 1.
Tissue distribution of podocan in mouse and human tissues. (a–c) Northern blot analysis of mouse podocan mRNA in the liver (Li), lung (Lu), stomach (St), kidney (K), brain (Br), small intestine (I), heart (H), testis (T), epididymal WAT (E), mesenteric WAT (M), and brown adipose tissue (Ba) of 8-week-old C57BL/6J mice (n = 4) using two different DIG-labeled antisense podocan RNA probes. (d) The tissue distribution of human podocan mRNA in brain-cerebellum (brain-cere), whole brain, fetal brain, fetal liver, heart, kidney, liver, lung, placenta, prostate, salivary gland (salivary-g), skeletal muscle (SKM), spleen, testis, thymus, thyroid, trachea, uterus, colon mucosa (colon-mu), small intestine (small-int), hypothalamus (hypothala), skin, melanocyte, and adipocyte were analyzed by quantitative RT-PCR.
C. Northern Blotting
Male C57BL/6J mice (n = 4) were purchased from CLEA Japan (Tokyo, Japan). At 8 weeks of age, the mice were euthanized by bleeding the vena cava under isoflurane anesthesia. Liver, lung, stomach, heart, kidney, testis, small intestine, brain, brown adipose tissue, mesenteric white adipose tissue (WAT), and epididymal WAT were harvested and immersed in RNAlater® RNA Stabilization Solution (Ambion, Tokyo, Japan). Total RNA was extracted from these organs using RNA STAT-60 reagent (Tel-Test, Friendswood, TX). RNA (10 μg) was loaded onto a 1.3% agarose gel and transferred to a nylon membrane (Hybond N+; GE Health Care Life Sciences, Tokyo, Japan). RNA molecular weight marker-1 (11526529910; Roche Diagnostics, Basel, Switzerland) was used to determine the molecular weight of RNA on the northern blots. DIG-labeled antisense podocan RNA probes were used to detect the podocan mRNA. The signal was developed using the chemiluminescent substrate CDP-Star (Roche Diagnostics), as per manufacturer’s instructions.
D. Tissue Distribution of Podocan in Humans
Human total RNA master panel 2 (636643; Clontech, Mountain View, CA), human skin total RNA (R1234218-P; BioChain, Newark, CA), human melanocyte total RNA (104-R10n; Cell Applications, San Diego, CA), human hypothalamus Poly A+ RNA (636144; Clontech), and human adipose tissue total RNA (636558; Clontech) were used to determine the tissue distribution of podocan mRNA in humans. Complementary DNA (cDNA) for real-time quantitative polymerase chain reaction (PCR) analysis was synthesized from total RNA using a high-capacity cDNA reverse transcription (RT) kit (Applied Biosystems, Carlsbad, CA) according to manufacturer’s instructions.
E. Animals
Male C57BL/6J mice, KKAy mice, BKS.Cg-+Leprdb/+Leprdb (db/db) mice, and BKS.Cg-m+/+Leprdb (db/+) mice were purchased from CLEA Japan. Male B6.V-Lepob/J, ob/ob mice were purchased from Charles River (Yokohama, Japan). To generate diet-induced obese (DIO)-C57BL/6J mice, male C57BL/6J mice were individually housed and fed with a high-fat diet (D12451; CLEA Japan) for 46 weeks from 6 weeks of age. All mice except the DIO-C57BL/6J mice were fed with a standard diet (CE-2; CLEA Japan) and tap water ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee of Shonan Research Center, Takeda Pharmaceutical Company.
F. Pathological Changes in Podocan mRNA Levels in Epididymal WAT of Diabetic Mice
Male C57BL/6J (18-week-old), KKAy (22-week-old), ob/ob (16-week-old), and db/db (16-week-old) mice at the ad libitum state (n = 5 for each group) were weighed and their blood glucose levels measured using blood drawn from the tail vein (Glutest Sensor-PRO; Sanwa Chemical, Tokyo, Japan). Plasma glucose (PG) values over 600 mg/dL were calculated as 600 mg/dL because of sensor limitation. The mice were anesthetized by 2% isoflurane. Then, epididymal WAT and small intestine were dissected. They were immersed in RNAlater® RNA stabilization reagent (Qiagen, Hilden, Germany). Podocan mRNA levels in epididymal WAT and intestine and tumor necrosis factor (TNF)-α mRNA in epididymal WAT were measured by quantitative RT-PCR.
G. Podocan mRNA Expression in Differentiated 3T3-L1 Adipocytes
Mouse 3T3-L1 preadipocytes were purchased from DS-Pharma (Osaka, Japan) and were cultured in Dulbecco’s modified Eagle medium (DMEM) containing 25 mM glucose (11965; GIBCO, Grand Island, NY) and 10% fetal bovine serum (FBS) (10099; GIBCO) at 37°C in 5% CO2. The cells were seeded into six-well culture plates (400,000 cells/well) (3516; Corning, NY). Confluent cultures were induced to differentiate into adipocytes by incubation in DMEM containing 25 mM glucose, 10% FBS, 0.25 units/mL insulin (11061; WAKO Pure Chemical Industries, Osaka, Japan), 0.25 nM dexamethasone (047-18863; WAKO), and 0.5 mM isobutyl-1-methylxanthine (095-03413; WAKO). After 2 days, the medium was changed to DMEM containing 25 mM glucose, 10% FBS, and 0.25 units/mL insulin with 0, 1, 10, or 100 nM TNF-α (3410; R&D Systems) and incubated for 5 days at 37°C in 5% CO2 for adipocyte differentiation. These cells were harvested and subjected to total RNA extraction by the RNeasy Mini Kit for use in real-time quantitative PCR analysis.
H. Detection of Plasma Podocan Secretion and Changes in Plasma Podocan Levels in DIO-C57BL/6J and Obese-Diabetic KKAy Mice
Male C57BL/6J (10-week-old), DIO-C57BL/6J (52-week-old), and KKAy (22-week-old) mice (n = 5) were weighed, and their blood samples were obtained by facial vein puncture. Blood glucose levels were measured using the Glutest sensor (Sanwa Chemical). Plasma podocan levels were measured using the mouse podocan enzyme-linked immunosorbent assay (ELISA) kit (96186MU-WLS; Cloud-Clone Corp., Katy, TX).
I. Changes in Blood Glucose Levels After Injection of Recombinant Podocan Protein in KKAy Mice
Recombinant mouse podocan protein (3104-PO; R&D Systems) was reconstituted in phosphate-buffered saline (PBS). The body weight and blood glucose levels of male 24-week-old KKAy mice were measured. The mice were divided into three groups and administered PBS (10 mL/kg body weight), podocan (50 μg/0.5 mL/head), and insulin (2 U/10 mL/kg body weight) by intraperitoneal injection (n = 5 per group). Blood glucose concentrations were measured before or after injection at 15, 30, 60, 180, and 300 minutes as indicated.
J. Podocan mRNA Levels in the Renal Cortex and Plasma Podocan Levels in db/+ Mice and Uninephrectomized-db/db Mice
Blood samples were obtained by facial vein puncture in male 28-week-old db/+ and uninephrectomized (UNx)-db/db mice that received unilateral nephrectomy at the age of 6 weeks. Plasma samples were separated by centrifugation, and PG levels were measured with the Autoanalyzer 7180 (Hitachi, Japan). Urine samples were collected over 8 hours using metabolic cages, and urine creatinine levels were measured with the Autoanalyzer 7180. After desalination of the urine samples using the PD MiniTrap G-25 column (GE Health Care, United Kingdom), urinary albumin levels were measured by ELISA (Shibayagi, Tokyo, Japan), and the urinary albumin-to-creatinine ratio (UACR) was calculated. These mice were then anesthetized using 2% isoflurane inhalation, and their remaining left kidneys were dissected, weighed, and processed for histopathological analysis using hematoxylin and eosin staining. The renal cortex was immersed in RNAlater® RNA stabilization reagent. The podocan mRNA levels were measured by quantitative RT-PCR. The plasma podocan levels were measured using the mouse podocan ELISA kit (Cloud-Clone Corp., Katy, TX).
K. Correlation of Renal Podocan mRNA Levels With Amelioration of Diabetic Nephropathy
Male db/db mice were subjected to unilateral nephrectomy at the age of 6 weeks to generate UNx-db/db mice. After a 12-week recovery period from unilateral nephrectomy, blood samples were obtained by facial vein puncture from age-matched db/+ and UNx-db/db mice. The level of glycosylated hemoglobin (GHb) in blood was measured by an automated high-performance liquid chromatography–based GHb analyzer (HLC-723 G8; TOSOH, Tokyo, Japan). PG levels were measured using the Autoanalyzer 7180. Urine samples were collected over 8 hours using metabolic cages, and the UACR was calculated. UNx-db/db mice were divided into four groups according to body weight, UACR, PG, and GHb levels. Irbesartan (5, 20, and 50 mg/kg quaque die), an angiotensin II receptor blocker, was suspended in 0.5% methylcellulose solution and was orally administered to mice for 8 weeks from 18 weeks of age. After the 8-week repeated dose, blood, plasma, and urine samples were obtained using the same methods as described above. Body weight, PG, and UACR were measured. These mice were then anesthetized using 2% isoflurane inhalation, and their remaining left kidneys were dissected and weighed. The renal cortex was immersed in RNAlater® RNA stabilization reagent. Podocan and renin 1 mRNA levels were then measured by quantitative RT-PCR.
L. mRNA Expression Analysis by Quantitative Real-Time PCR in Human Mesangial Cells
Human mesangial cells were purchased from DS Pharma Biomedical (Osaka, Japan). On day 0, these cells were seeded at a density of 2 × 105 cells/well in Cell Systems Corporation (CSC) complete medium (Cell Systems Corporation, Kirkland, WA) on a type 1, collagen-coated, 24-well plate (AGC Techno Glass Co. Ltd., Tokyo, Japan) in an atmosphere of 95% air and 5% CO2 at 37°C. On day 1, the cells were serum starved in CSC medium lacking serum and growth factors. On day 2, recombinant human podocan protein (TP305567; OriGene Technologies, Rockville, MD) was reconstituted in PBS. Podocan, at final concentrations of 0, 1, 10, 100, and 1000 ng/mL with TGF-β (final concentration of 1 ng/mL) or PBS (vehicle, without TGF-β), was added to the culture medium. After 24 hours, the medium was removed and cells were lysed in the lysis buffer of the RNeasy 96 Kit (Qiagen). Total RNA was purified by the RNeasy 96 Kit according to the manufacturer’s instruction. cDNA was synthesized by RT reaction (high-capacity cDNA RT kit; Life Technologies, Waltham, MA). mRNA levels of type 1 collagen α1 chain (collagen 1A1), fibronectin, connective tissue growth factor (CTGF), and plasminogen activator inhibitor-1 (PAI-1), were measured by quantitative real-time RT-PCR.
M. Quantitative RT-PCR
Total RNA was isolated from mouse tissues and 3T3-L1 preadipocytes and adipocytes using the RNeasy Mini Kit (Qiagen). cDNA was synthesized from total RNA using a high-capacity cDNA RT kit (Applied Biosystems, Waltham, MA) for quantitative real-time PCR analysis. Predesigned primers and probes (Applied Biosystems) were used for the quantification of gene expression for the following genes: human podocan, Hs_00542259m1; human collagen 1A1, Hs_00164004_m1; human fibronectin, Hs_00365052_m1; human CTGF, Hs_01026927_g1; human PAI-1, Hs_00167155_m1; mouse podocan, Mm00556334_m1; mouse TNF-α, Mm00443258_m1; mouse renin-1, Mm02342887_mH; mouse glyceraldehyde-3-phosphate dehydrogenase, Mm99999915_g1; and human cyclophilin, Hs00332817_m1. The mRNA levels were quantified using an ABI Prism 7900 PCR system (Applied Biosystems). The relative expression of each transcript was normalized to the expression of human cyclophilin or mouse glyceraldehyde-3-phosphate dehydrogenase mRNA.
N. Statistical Analysis
Results are expressed as the mean ± standard deviation (SD). Differences between two groups were assessed using the Student t test or Aspin-Welch t test; differences between more than two groups such as the dose-dependent studies were assessed using the one-tailed Williams test. For time course analysis of blood glucose change after injection of recombinant podocan protein, differences were assessed using the Student t test or Aspin-Welch t test followed by Bonferroni’s correction for comparing multiple time points vs the control group.
2. Results
A. Tissue Distribution of Podocan in Mice and Humans
Previous studies have reported that podocan is expressed in injured kidney and in the cardiovascular system. However, there are few reports describing the tissue distribution of podocan in mice and human. To explore the precise tissue distribution of podocan, we first performed northern blotting analysis on tissue-derived mRNA from normal C57BL/6J mice using two different probes [Fig. 1(a)]. Surprisingly, mouse podocan mRNA was highly expressed in epididymal WAT, brown adipose tissue, and small intestine [Fig. 1(b) and 1(c)]. The elevated expression of podocan mRNA in these tissues was confirmed by quantitative RT-PCR (Supplemental Fig. 1 (836.1KB, pptx) ). We also assessed podocan mRNA levels in human tissues by quantitative RT-PCR. Podocan mRNA was highly expressed in the human heart, adipose tissue, skeletal muscle, testis, trachea, and uterus [Fig. 1(d)]. As podocan was commonly expressed in human and mouse adipose tissue, we next focused on the expression of podocan in WAT.
B. Pathological Changes Associated With Podocan mRNA Levels in Epididymal WAT in Diabetic Mice, and the Effect of TNF-α on Podocan mRNA in 3T3-L1 Adipocytes
To determine the effect of diabetes on podocan mRNA levels, we compared podocan mRNA levels in the epididymal WAT between normal C57BL/6J mice and type 2 diabetic and obese mice such as KKAy mice, ob/ob mice, and db/db mice. The body weight and blood glucose levels of these three mice were significantly higher compared with those of normal C57BL/6J mice [Fig. 2(a) and 2(b)]. Interestingly, the podocan mRNA levels in the epididymal WAT of these diabetic mice were suppressed significantly compared with those in normal C57BL/6J mice [Fig. 2(c)]. In contrast, TNF-α mRNA level in epididymal WAT was significantly elevated in the diabetic mice [Fig. 2(d)]. However, there were almost no changes in podocan mRNA expression in the small intestine between the different types of mice [Fig. 2(e)]. To explore the correlation between podocan and TNF-α expression, we analyzed the changes in podocan mRNA levels upon addition of TNF-α in 3T3-L1 adipocyte culture. TNF-α treatment significantly suppressed podocan expression in 3T3-L1 adipocytes [Fig. 2(f)]. We also measured podocan mRNA in 3T3-L1 preadipocytes and differentiated adipocytes (7 days after stimulation of differentiation). There was a significant decrease of podocan mRNA expression in 3T3-L1 cells upon differentiation (Supplemental Fig. 2 (836.1KB, pptx) ).
Figure 2.
Effect of diabetes on podocan mRNA expression in epididymal (Epi) WAT and the effect of TNF-α on podocan mRNA levels in 3T3-L1 adipocytes. (a) Body weight and (b) blood glucose levels of male 18-week-old C57BL/6J mice (n = 5), 22-week-old KKAy mice (n = 5), 16-week-old ob/ob mice (n = 5), and 16-week-old db/db mice (n = 5) at ad libitum state were measured. Podocan mRNA and TNF-α mRNA levels in epididymal WAT (c and d) and podocan mRNA level in the intestine (e) were measured by quantitative RT-PCR (n = 5 per group). (f) Podocan mRNA levels in differentiated 3T3-L1 adipocytes were measured after treatment with 0, 1, 10, and 100 nM TNF-α for 5 days. Each experiment was repeated at least twice. Data are expressed as the mean ± SD. *P < 0.05 compared with C57BL/6J mice by Student t test or Aspin-Welch t test; #P ≤ 0.025 compared with vehicle by one-tailed Williams test.
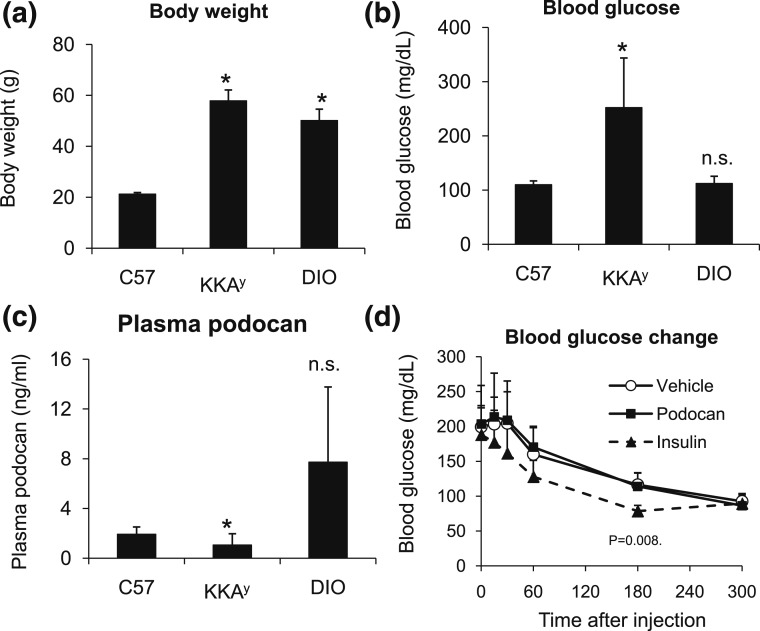
C. Podocan Secretion in the Plasma of DIO-C57BL/6J and Obese-Diabetic KKAy Mice and Changes in Blood Glucose Levels After Injection of Recombinant Podocan in KKAy Mice
Podocan has signal peptide sequences at its N-terminal region and is secreted in the cell culture supernatants of both HIV-1 transgenic podocytes and HEK-293T cells [2]. Thus, using C57BL/6J mice, KKAy mice, and DIO-C57BL/6J mice, we aimed to determine whether podocan is secreted into the blood under normal and obese-diabetic conditions. The body weights of KKAy and DIO-C57BL/6J mice were elevated significantly [Fig. 3(a)], and the blood glucose level of KKAy mice was significantly high [Fig. 3(b)] compared with the C57BL/6J mice. As expected, podocan was secreted in the plasma; the plasma concentrations were similar to those of leptin (ng/mL) and were significantly suppressed in the diabetic condition, as in KKAy mice. In contrast, plasma podocan concentration tended to increase in the DIO-C57BL/6J mice [Fig. 3(c)]. According to these results, the reduction of plasma podocan concentration may have a correlation with changes in the blood glucose level. To confirm this notion, we performed podocan complementary therapy in KKAy mice by intraperitoneal injection of podocan recombinant protein (50 μg). Interestingly, the administration of podocan did not affect the blood glucose levels in KKAy mice [Fig. 3(d)]. As leptin and leptin receptor deficiencies in the ob/ob and db/db mice, respectively, could interfere with the effects of podocan administration, we restricted this study to the KKAy mice only.
Figure 3.
Detection of podocan secretion in the plasma in DIO-C57BL/6J mice and in obese-diabetic KKAy mice, and changes in blood glucose after injection of recombinant podocan protein in KKAy mice. (a) Body weight, (b) blood glucose levels, and (c) plasma podocan levels of male 10-week-old C57BL/6J mice (n = 5), 52-week-old DIO-C57BL/6J mice (n = 5), and 22-week-old KKAy mice (n = 5) were measured. The 24-week-old KKAy male mice were divided into three groups based on body weight and blood glucose levels and were administered PBS (10 mL/kg of body weight), podocan (50 μg/head/0.5 mL), and insulin (2 U/10 mL/kg of body weight) by intraperitoneal injection (n = 5 per group). (d) Blood glucose levels were measured before or after the injection at the indicated time points. Data are expressed as the mean ± SD. *P < 0.05 compared with C57BL/6J mice or vehicle treatment by Student t test or Aspin-Welch t test, followed by Bonferroni’s correction for comparing multiple time points vs the control group.
D. Correlation of Renal Podocan mRNA Levels With Diabetic Nephropathy
We evaluated the correlation of renal podocan mRNA levels with diabetic nephropathy using UNx-db/db mice, a mouse model of obesity and type 2 diabetes mellitus, complicated with diabetic nephropathy. The body weight, kidney weight, PG levels, and UACR in these mice were significantly elevated compared with normal db/+ mice [Fig. 4(a–d)]. On the contrary, serum creatinine levels of UNx-db/db mice (5.1 ± 1.6 mg/dL) were low compared with those of the normal db/+ mice (39.4 ± 5.7 mg/dL). Podocan mRNA levels in the renal cortex and the plasma podocan levels were significantly decreased compared with that of the normal db/+ mice [Fig. 4(e) and 4(f)]. We conducted a histological study in the kidney of db/+ mice and db/db mice. There was a significant expansion of the mesangial matrix in the glomeruli of UNx-db/db mice as compared with the db/+ mice (Supplemental Fig. 3 (836.1KB, pptx) ). We also evaluated the blood pressure of UNx-db/db mice; their mean blood pressure was 114.8 ± 9.5 mm Hg, which falls within the normal range.
Figure 4.
Podocan mRNA levels in the renal cortex and plasma podocan levels in db/+ mice and UNx-db/db mice. Body weights of male 28-week-old db/+ mice (n = 5) and UNx-db/db mice (n = 6) (a) and (b) PG levels were measured. (c) Urine samples were collected over 8 hours using metabolic cages, and the UACR was calculated. (d) Kidney weights, (e) podocan mRNA levels in the renal cortex, and (f) plasma podocan concentrations of these mice were measured. Data are expressed as the mean ± SD. *P < 0.05 compared with db/+ mice by Student t test or the Aspin-Welch t test.
E. Correlation of Renal Podocan mRNA Levels With Amelioration of Diabetic Nephropathy
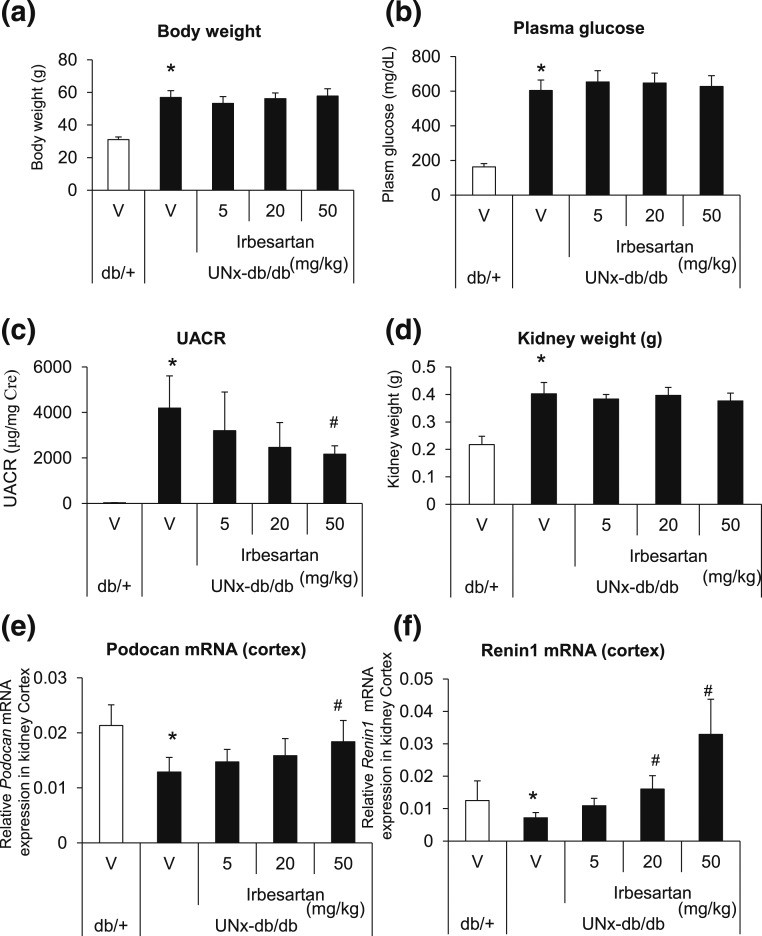
To confirm the relationship between diabetic nephropathy and podocan expression in the kidney, we further evaluated the changes in podocan mRNA levels in the kidney of UNx-db//db mice with the amelioration of diabetic nephropathy using irbesartan treatment. Irbesartan treatment significantly suppressed elevation of UACR in a dose-dependent manner, without having significant effects on the body weight, kidney weight, and PG levels [Fig. 5(a–d)]. Similar to previous studies [Fig. 4(e)], the podocan mRNA levels in the renal cortex were significantly decreased in UNx-db/db mice compared with in normal db/+ mice, and were significantly improved by irbesartan treatment [Fig. 5(e)]. A similar trend was observed in the mRNA levels of renin 1, a pharmacodynamic marker of angiotensin II receptor blockade [Fig. 5(f)].
Figure 5.
Correlation of renal podocan mRNA levels with amelioration of diabetic nephropathy. UNx-db/db mice at 18 weeks of age were orally administered the vehicle (0.5% methyl cellulose solution) or irbesartan (5, 20, and 50 mg/kg) every day for 8 weeks (n = 5 per group). Age-matched db/+ mice (n = 10) were also orally administered the vehicle every day for 8 weeks. (a) Body weight, (b) PG levels, and (c) UACR were measured. Then their kidneys were dissected, and (d) kidney weight, (e) podocan mRNA levels, and (f) renin 1 mRNA levels in the renal cortex were measured. Data are expressed as the mean ± SD. *P < 0.05 compared with the db/+ mice by Student t test or Aspin-Welch t test; #P ≤ 0.025 compared with the vehicle by one-tailed Williams test.
F. Effect of Podocan on TGF-β–Induced Profibrotic Gene Expression in Human Mesangial Cells
To evaluate whether podocan expression is affected by profibrotic stimulation, we stimulated human mesangial cells with TGF-β and measured podocan mRNA levels. The levels of podocan mRNA was slightly, but significantly, elevated by TGF-β stimulation in human mesangial cells [Fig. 6(a)]. Then, we analyzed the antifibrotic effect of podocan after TGF-β stimulation in these cells. Podocan slightly reduced the expression of profibrotic genes such as collagen 1A1, fibronectin, and CTGF [Fig. 6(b–d)]; however, there was almost no change in the expression of the PAI-1 mRNA [Fig. 6(e)]. These results suggest that podocan has mild suppressive effects on promotion of fibrosis but does not cause fibrinolysis on this cell type.
Figure 6.
Effect of podocan on TGF-β–induced profibrotic gene expression in human mesangial cells. Human mesangial cells were seeded on a type 1, collagen-coated, 24-well plate on day 0. On day 1, the cells were serum starved with CSC medium. On day 2, recombinant human podocan at final concentrations of 0, 1, 10, 100, and 1000 ng/mL with TGF-β (final concentration of 1 ng/mL) or PBS (vehicle, without TGF-β) was added to the culture medium. The cells were lysed after 24 hours. mRNA levels of (a) podocan, (b) collagen 1A1, (c) fibronectin, (d) CTGF, (e) and PAI-1 were measured by quantitative real-time RT-PCR. Data are expressed as the mean ± SD (n = 4 per treatment group). *P < 0.05 compared with the db/+ mice by Student t test or Aspin-Welch t test; #P ≤ 0.025 compared with the vehicle by one-tailed Williams test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
3. Discussion
In this study, we found that podocan is highly expressed in both mouse and human adipose tissues and also present at detectable levels in the mouse plasma. Our findings in this paper further show that podocan mRNA is negatively regulated by TNF-α stimulation in 3T3-L1 adipocytes. As reported in previous studies [16, 17], TNF-α mRNA levels were significantly elevated in WAT of KKAy mice. Thus, the reduction of podocan mRNA in the WAT of KKAy mice might result from an increase in TNF-α from WAT.
The plasma podocan level increased in DIO-C57BL/6J mice but decreased in KKAy and db/db mice. In this study, there were individual differences in plasma podocan levels of DIO-C57BL/6J mice. It would be beneficial to increase the sample size of DIO-C57BL/6J mice to obtain more precise data of plasma podocan levels in DIO-C57BL/6J mice. Plasma insulin levels have been reported to increase to a greater extent in obese-diabetic mice such as the db/db mice than in obese DIO-C57BL/6J mice without diabetes [18]. In adipose tissues, TNF-α mRNA levels are elevated, which correlates highly with plasma insulin levels [19]. In our study, the differences observed in plasma insulin levels between DIO-C57BL/6J mice, KKAy mice, and db/db mice may correlate with TNF-α mRNA levels in WAT. This may lead to differences in the plasma podocan levels of these mice. It would be interesting to examine these obese-diabetic mice over an extended period to determine if podocan expression reduces with the gradual development of obesity, thus providing further understanding of the regulation of podocan in adipose tissue. Moreover, it would be of interest to follow podocan expression at different ages to see if the reduced expression develops with obesity development. Further mechanistic analysis is required to determine the precise function of podocan in adipose tissue.
The levels of decorin mRNA, which showed similar regulation of expression as that of podocan, decreased with the progression of nephropathy symptoms such as proteinuria [9, 13, 20–24]. According to the phenotypes of diabetic decorin-deficient mice, decorin showed renal protective effect by inhibition of the TGF-β signaling pathway [25–27]. Thus, we speculated that podocan might show renal protective effects during the development of diabetic nephropathy. To confirm this hypothesis, we evaluated the changes in podocan mRNA levels in the kidney after repeated dosing of irbesartan to UNx-db/db mice. UACR increased significantly in the UNx-db/db mice. In contrast, the podocan mRNA levels in the renal cortex of UNx-db/db mice were significantly decreased. After 8 weeks of repeated dosing of irbesartan, a significant dose-dependent suppression of elevated UACR levels was observed in the UNx-db/db mice [28, 29]. In contrast, the podocan mRNA levels in the cortex decreased in the UNx-db/db mice [30, 31]. The ideal control for studying the UNx-db/db mice would be the UNx db/+ mice, which we were unable to generate. Nonetheless, our findings suggest that podocan expression correlates negatively with UACR levels and glomerular injury [1, 5, 32]. Future studies will focus on evaluating the link between circulating podocan and diabetic kidney disease. To this end, we will generate whole or tissue-specific podocan knockout mice. To explore the precise role of podocan in diabetic nephropathy, we will evaluate the effect of neutralizing podocan with an anti-podocan antibody, as well as podocan knockdown on urine albumin and serum creatinine in a murine model of diabetic nephropathy. Our study showed that podocan mRNA levels were increased by TGF-β stimulation, and podocan treatment mildly suppressed TGF-β–induced profibrotic gene expression in human mesangial cells. Therefore, podocan may play an antifibrotic role in the glomeruli, similar to decorin.
In this study, we found detectable levels of podocan in the plasma. Plasma podocan levels decreased significantly in obese-diabetic mice such as the KKAy mice. Intraperitoneal injection of recombinant podocan into KKAy mice did not affect the PG levels in these mice. Thus, podocan might not directly affect glucose metabolism, in contrast to leptin [33–35] and adiponectin [36–38]. Therefore, the function of circulating podocan remains unknown. In conclusion, we found that podocan was highly expressed in human and mouse adipose tissue and that podocan expression was regulated by TNF-α in 3T3-L1 adipocytes.
In kidneys of the UNx-db/db mice, podocan expression correlated with glomerular injury, as did the UACR levels. Furthermore, podocan was secreted into the plasma and plasma podocan levels were affected in obesity and diabetic conditions. Future studies will be conducted to determine the source of plasma podocan. To this end, we will measure the concentrations of podocan in the medium of cultured adipocytes, muscle cells, hepatocytes, and kidney cells. Although the precise mechanisms underlying the effects of podocan on obesity, diabetes, and diabetic nephropathy are unknown, complementary podocan therapy using podocan-deficient mice as models of cardiovascular disorders, diabetes, and diabetic nephropathy would provide important information for future studies.
Acknowledgments
We thank Masatoshi Hazama for important advice and comments regarding this study.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cDNA
- complementary DNA
- collagen 1A1
- type 1 collagen α1 chain
- CTGF
- connective tissue growth factor
- DIG
- digoxigenin
- DIO
- diet-induced obese
- DMEM
- Dulbecco’s modified Eagle medium
- ELISA
- enzyme-linked immunosorbent assay
- FBS
- fetal bovine serum
- GHb
- glycosylated hemoglobin
- mRNA
- messenger RNA
- ORF
- open reading frame
- PAI-1
- plasminogen activator inhibitor-1
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PG
- plasma glucose
- RT
- reverse transcription
- SD
- standard deviation
- SLRP
- small leucine-rich repeat proteoglycan
- SMC
- smooth muscle cell
- TGF
- transforming growth factor
- TNF
- tumor necrosis factor
- UACR
- urinary albumin-to-creatinine ratio
- Unx
- uninephrectomized
- WAT
- white adipose tissue.
References and Notes
- 1.Shimizu-Hirota R, Sasamura H, Kuroda M, Kobayashi E, Saruta T. Functional characterization of podocan, a member of a new class in the small leucine-rich repeat protein family. FEBS Lett. 2004;563(1-3):69–74. [DOI] [PubMed] [Google Scholar]
- 2.Ross MD, Bruggeman LA, Hanss B, Sunamoto M, Marras D, Klotman ME, Klotman PE. Podocan, a novel small leucine-rich repeat protein expressed in the sclerotic glomerular lesion of experimental HIV-associated nephropathy. J Biol Chem. 2003;278(35):33248–33255. [DOI] [PubMed] [Google Scholar]
- 3.Le Roux S, Devys A, Girard C, Harb J, Hourmant M. Biomarkers for the diagnosis of the stable kidney transplant and chronic transplant injury using the ProtoArray® technology. Transplant Proc. 2010;42(9):3475–3481. [DOI] [PubMed] [Google Scholar]
- 4.Signal peptide database. Available at: www.signalpeptide.de/. Accessed 15 May 2015.
- 5.Sun Y, Luo DY, Zhu YC, Zhou L, Yang TX, Tang C, Shen H, Wang KJ. MiR 3180-5p promotes proliferation in human bladder smooth muscle cell by targeting PODN under hydrodynamic pressure. Sci Rep. 2016;6:33042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutter R, Huang L, Speidl WS, Giannarelli C, Trubin P, Bauriedel G, Klotman ME, Fuster V, Badimon JJ, Klotman PE. Novel small leucine-rich repeat protein podocan is a negative regulator of migration and proliferation of smooth muscle cells, modulates neointima formation, and is expressed in human atheroma. Circulation. 2013;128(22):2351–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniel Katz. Podocan and wnt pathway in the development of aortopathy in bicuspid valve disease. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT02806310?term=Podocan&rank=3. Accessed 1 March 2017.
- 8.Bolton K, Segal D, McMillan J, Jowett J, Heilbronn L, Abberton K, Zimmet P, Chisholm D, Collier G, Walder K. Decorin is a secreted protein associated with obesity and type 2 diabetes. Int J Obes. 2008;32(7):1113–1121. [DOI] [PubMed] [Google Scholar]
- 9.Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol. 2009;60(Suppl 4):5–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer L, Raslik I, Grone HJ, Schonherr E, Macakova K, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J. 2001;15(3):559–561. [DOI] [PubMed] [Google Scholar]
- 11.Carroll JF, Tyagi SC. Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbit. Am J Hypertens. 2005;18(5 Pt 1):692–698. [DOI] [PubMed] [Google Scholar]
- 12.Ferrini MG, Moon J, Rivera S, Rajfer J, Gonzalez-Cadavid NF. Amelioration of diabetes-induced cavernosal fibrosis by antioxidant and anti-transforming growth factor-β1 therapies in inducible nitric oxide synthase-deficient mice. BJU Int. 2012;109(4):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng F, Lu W, Wu F, Li H, Hu X, Zhang F. Recombinant decorin ameliorates the pulmonary structure alterations by down-regulating transforming growth factor-β1/SMADS signaling in the diabetic rats. Endocr Res. 2010;35(1):35–49. [DOI] [PubMed] [Google Scholar]
- 14.Williams KJ, Qiu G, Usui HK, Dunn SR, McCue P, Bottinger E, Iozzo RV, Sharma K. Decorin deficiency enhances progressive nephropathy in diabetic mice. Am J Pathol. 2007;171(5):1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolton K, Segal D, Walder K. The small leucine-rich proteoglycan, biglycan, is highly expressed in adipose tissue of Psammomys obesus and is associated with obesity and type 2 diabetes. Biologics. 2012;6:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann C, Lorenz K, Braithwaite SS, Colca JR, Palazuk BJ, Hotamisligil GS, Spiegelman BM. Altered gene expression for tumor necrosis factor-α and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994;134(1):264–270. [DOI] [PubMed] [Google Scholar]
- 17.Fu L1, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y. The effects of β(3)-adrenoceptor agonist CL-316,243 on adiponectin, adiponectin receptors and tumor necrosis factor-α expressions in adipose tissues of obese diabetic KKAy mice. Eur J Pharmacol. 2008;584(1):202–206. [DOI] [PubMed] [Google Scholar]
- 18.Li E, Nakata M, Shinozaki A, Yang Y, Zhang B, Yada T. Betatrophin expression is promoted in obese hyperinsulinemic type 2 but not type 1 diabetic mice. Endocr J. 2016;63(7):611–619. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A, Eckardt K. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. 2014;450(2):1089–1094. [DOI] [PubMed] [Google Scholar]
- 21.Onuma H, Tabara Y, Kawamura R, Ohashi J, Nishida W, Takata Y, Ochi M, Nishimiya T, Kawamoto R, Kohara K, Miki T, Osawa H. Plasma resistin is associated with single nucleotide polymorphisms of a possible resistin receptor, the decorin gene, in the general Japanese population. Diabetes. 2013;62(2):649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel-Wahab N, Wicks SJ, Mason RM, Chantry A. Decorin suppresses transforming growth factor-β-induced expression of plasminogen activator inhibitor-1 in human mesangial cells through a mechanism that involves Ca2+-dependent phosphorylation of Smad2 at serine-240. Biochem J. 2002;362(Pt 3):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan H, Chen Y, Li L, Jiang J, Wu G, Zuo Y, Zhang JH, Feng H, Yan X, Liu F. Decorin alleviated chronic hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after subarachnoid hemorrhage in rats. Brain Res. 2016;1630:241–253. [DOI] [PubMed] [Google Scholar]
- 24.Alan C, Kocoglu H, Altintas R, Alici B, Resit Ersay A. Protective effect of decorin on acute ischaemia-reperfusion injury in the rat kidney. Arch Med Sci. 2011;7(2):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbah SA, Thomas D, Browne S, O'Brien T, Pandit A, Zeugolis DI. Co-transfection of decorin and interleukin-10 modulates pro-fibrotic extracellular matrix gene expression in human tenocyte culture. Sci Rep. 2016;6:20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Li J, Wang K, Zhang Z, Zhang W, Zhou G, Cao Y, Ye M, Zou H, Liu W. Induction of predominant tenogenic phenotype in human dermal fibroblasts via synergistic effect of TGF-β and elongated cell shape. Am J Physiol Cell Physiol. 2016;310(5):C357–C372. [DOI] [PubMed] [Google Scholar]
- 27.Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, Ishikawa Y, Weis MA, Sampath TK, Ambrose C, Eyre D, Bächinger HP, Lee B. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20(6):670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XW, Du XY, Wang YX, Wang JC, Liu WT, Chen WJ, Li HY, Peng FF, Xu ZZ, Niu HX, Long HB. Irbesartan ameliorates diabetic nephropathy by suppressing the RANKL-RANK-NF-κB pathway in type 2 diabetic db/db mice. Mediators Inflamm. 2016;2016:1405924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu QX, Zhang H, Xu WH, Hao F, Liu SL, Bai MM, Mu JW, Zhang HJ. Effect of irbesartan on chemerin in the renal tissues of diabetic rats. Kidney Blood Press Res. 2015;40(5):467–477. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi A, Fukusumi Y, Yamazaki M, Kayaba M, Kitazawa Y, Tomita M, Kawachi H. Angiotensin II type 1 receptor blockade ameliorates proteinuria in puromycin aminonucleoside nephropathy by inhibiting the reduction of NEPH1 and nephrin. J Nephrol. 2014;27(6):627–634. [DOI] [PubMed] [Google Scholar]
- 31.Bonnet F, Cooper ME, Kawachi H, Allen TJ, Boner G, Cao Z. Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia. 2001;44(7):874–877. [DOI] [PubMed] [Google Scholar]
- 32.Ortmann J, Amann K, Brandes RP, Kretzler M, Münter K, Parekh N, Traupe T, Lange M, Lattmann T, Barton M. Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension. 2004;44(6):974–981. [DOI] [PubMed] [Google Scholar]
- 33.Burgos-Ramos E, Canelles S, Frago LM, Chowen JA, Arilla-Ferreiro E, Argente J, Barrios V. Improvement in glycemia after glucose or insulin overload in leptin-infused rats is associated with insulin-related activation of hepatic glucose metabolism. Nutr Metab (Lond). 2016;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgos-Ramos E, Canelles S, Rodríguez A, Gómez-Ambrosi J, Frago LM, Chowen JA, Frühbeck G, Argente J, Barrios V. Chronic central leptin infusion modulates the glycemia response to insulin administration in male rats through regulation of hepatic glucose metabolism. Mol Cell Endocrinol. 2015;415:157–172. [DOI] [PubMed] [Google Scholar]
- 35.Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, Nakao K. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001;50(6):1440–1448. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Kim DS, Kwon DY, Yang HJ. Long-term central infusion of adiponectin improves energy and glucose homeostasis by decreasing fat storage and suppressing hepatic gluconeogenesis without changing food intake. J Neuroendocrinol. 2011;23(8):687–698. [DOI] [PubMed] [Google Scholar]
- 37.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108(12):1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindberg S, Jensen JS, Bjerre M, Pedersen SH, Frystyk J, Flyvbjerg A, Mogelvang R. Cardio-adipose tissue cross-talk: relationship between adiponectin, plasma pro brain natriuretic peptide and incident heart failure. Eur J Heart Fail. 2014;16(6):633–638. [DOI] [PubMed] [Google Scholar]