Abstract
Complications associated with intraductal papillary mucinous neoplasms, such as acute pancreatitis, perforation, and fistula formation, have been documented. Intraductal papillary mucinous neoplasm with intratumoral hemorrhage is rare. To the best of our knowledge, there have been no previous reports of intraductal papillary mucinous neoplasm rupture and bleeding with intra-abdominal hemorrhage. A 74-year-old woman complained of acute upper right abdominal pain. She was under follow-up for an intraductal papillary mucinous neoplasm in the pancreatic head. Contrast-enhanced computed tomography revealed intraductal papillary mucinous neoplasm rupture and bleeding with intra-abdominal hemorrhage. The bleeding was treated with selective endovascular embolization of a branch of the gastroduodenal artery. Follow-up examinations are recommended even for intraductal papillary mucinous neoplasm patients without malignant findings because of the potential risk of rupture and bleeding with intra-abdominal hemorrhage. Clinicians should be aware of this possibility to ensure that patients are appropriately treated.
Keywords: Intraductal papillary mucinous neoplasm, bleeding, rupture, intra-abdominal bleeding, embolization
Introduction
Intraductal papillary mucinous neoplasm (IPMN) is characterized by the production of mucinous fluid by the tumor cells and tends to extend into a pancreatic duct. Development of imaging techniques and clinical advancements have led to more frequent recognition of IPMNs. Recently, IPMN of the pancreas has been found to be highly prevalent in the general population, and these tumors account for 8%–20% of all resected pancreatic neoplasms.1–3 IPMNs may be left untreated, but some may progress to invasive cancer (i.e. transform from a benign to a malignant tumor).1–3
We present a case of life-threatening spontaneous rupture of an IPMN, causing massive bleeding that appeared during follow-up in a patient with a pancreatic IPMN. Although acute pancreatitis, perforation, and fistula formation of pancreatic IPMNs have been reported,4–7 to the best of our knowledge, there have been no previous reports of IPMN rupture, causing life-threatening bleeding.
Case presentation
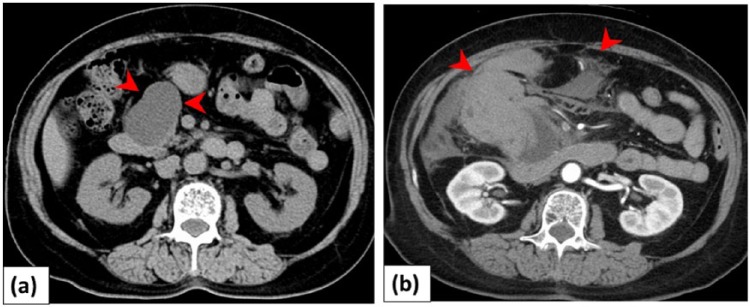
A 74-year-old woman complained of acute upper right abdominal pain and became unconscious. She had been followed every 6 months for an IPMN in the pancreatic head (Figure 1(a)), with no mural nodules and no changes in the size of the tumor over the past 5 years on abdominal ultrasonography and computed tomography (CT). She had no history of acute pancreatitis, and was not on medication including anticoagulant drugs. Levels of serum tumor markers (carcinoembryonic antigen and carbohydrate antigen 19-9) were within normal limits.
Figure 1.
Patient with intraductal papillary mucinous neoplasm (IPMN) and intra-abdominal bleeding: (a) a 74-year-old woman was followed for IPMN in the pancreatic head (arrowheads). The size did not change for 5 years. (b) Contrast-enhanced computed tomography showed IPMN rupture and intra-abdominal bleeding (arrowheads).
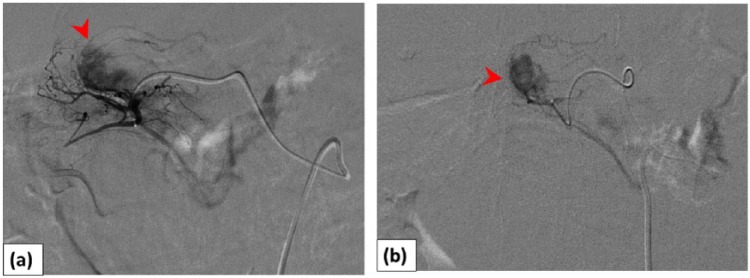
The abdomen was distended, and the shock index (defined as heart rate/systolic blood pressure) was 1.5 (blood pressure, 87/42 mm Hg; pulse 130 beats/min). The hemoglobin level was 6.1 g/dL. She became hemodynamically unstable with hypotension. Contrast-enhanced CT on admission revealed a massive hematoma spreading into the abdominal cavity, suggestive of an IPMN rupture. The main pancreatic duct was not dilated on CT (Figure 1(b)). Transfusion of packed red blood cells was followed by fluid overload. As her blood pressure was unstable, transarterial embolization (TAE) was initially selected. A 1.8-Fr coaxial microcatheter was advanced near the bleeding point of a branch of the gastroduodenal artery (GDA). TAE was performed using a gelatin sponge (Figure 2). After TAE, the patient became hemodynamically stable. Pylorus-preserving pancreaticoduodenectomy was performed 7 days later. During the operation, a ruptured cystic mass with hematoma was seen in the pancreatic head, and the tumor was completely removed with tumor-free margins (Figure 3(a)).
Figure 2.
Digital subtraction angiography images: (a) arteriography of a gastroduodenal artery (GDA) branch (superior pancreaticoduodenal artery) demonstrated tumor bleeding (arrowhead) and (b) a microcatheter was advanced near the bleeding point of the posterior superior pancreaticoduodenal artery (arrowhead). Embolization was performed using a gelatin sponge.
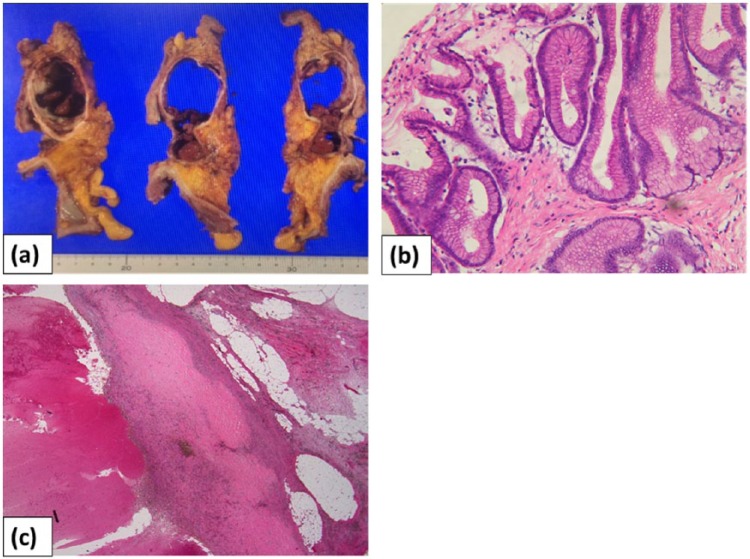
Figure 3.
Macroscopic and pathological findings: (a) macroscopic observations revealed that the resected cystic mass comprised a dark red, partially organized hematoma; (b) pathological examination revealed a growing papillary tumor characterized by low-grade dysplasia in the intrapancreatic duct; and (c) focal infiltration of hemosiderin-laden macrophages was observed in the pancreatic parenchyma.
Upon microscopic examination, the resected specimen showed a growing papillary tumor characterized by low-grade dysplastic cells in the intrapancreatic duct (Figure 3(b)), with a hematoma due to fresh and old bleeding. Focal infiltration of hemosiderin-laden macrophages was observed in the pancreatic parenchyma (Figure 3(c)). The final diagnosis was gastric-type IPMN with low-grade dysplasia, with intravascular emboli of the carcinoma type. The patient had an uneventful postoperative recovery without complications. In the approximately 2 years since the operation, there has been no sign of recurrence.
Discussion
IPMN of the pancreas is a distinct entity and is characterized by the proliferation of mucin-producing papillary epithelial cells, resulting in excessive mucus production and cystic dilatation of the pancreatic ducts. Various complications associated with IPMNs have been reported, including acute pancreatitis, intraductal bleeding, duct perforation, and fistula formation into the duodenum, common bile duct, and stomach.4–9 Even a non-malignant tumor may carry the risk of spontaneous rupture or bleeding.
The MEDLINE database was used to search for all English-language articles describing IPMNs in the pancreas complicated by bleeding or pancreatic duct rupture, and all relevant references cited in the identified articles were reviewed (Table 1).1,6,10–15 In all cases, the size of the tumor was greater than 20 mm, or the duct dilation was greater than 10 mm. There was no case with both intraductal bleeding and rupture of the pancreatic duct.
Table 1.
Case reports of intraductal papillary mucinous neoplasms (IPMNs) in the pancreas complicated with intraductal bleeding pancreatic duct rupture.
| Age/sex | Site of tumor | Size (cm) | Duct dilation | Symptom | Operation | Histological diagnosis | Rupture | Bleeding | |
|---|---|---|---|---|---|---|---|---|---|
| Zanelli et al.10 | 49/M | Diffuse & RP | 6 | NS | AP | PPPD | NS | (+) Pseudomyxoma peritonei | (−) |
| Mizuta et al.11 | 53/M | Tail | 2 | Mild | AF, LOA | Omental excision | NS | (+) Pseudomyxoma peritonei | (−) |
| Imaoka et al.12 | 64/M | Tail | NS | Severe | AP | DP | IV | (+) Pseudomyxoma peritonei | (−) |
| Lee et al.6 | 55/M | Body/tail | 8.5 | NS | None | DP | IV | (+) Pseudomyxoma peritonei | (−) |
| Nepka et al.13 | 82/M | NS | NS | 20 mm | Ascites | None | NS | (+) Pseudomyxoma peritonei | (−) |
| Rosenberger et al.14 | 75/M | Tail | 20 | 14.1 mm | None | DP | Moderate dysplasia | (+) Focally | (−) |
| 75/M | Head | 3.5 | 10 mm | None | PPPD | IV | (+) Focally | (−) | |
| Nagano et al.15 | 71/M | Head | 3.5 | NS | LOA | PPPD | IV | (+) Biliopancreatic fistula | (−) |
| Yamada et al.1 | 65/M | Head | NS | 38 mm | AP | PPPD | IPMA | (−) | (+) Intraductal |
| 60/M | Tail | NS | 28 mm | AP | DP | IPMA | (−) | (+) Intraductal | |
| 73/M | Head | NS | 30 mm | None | PPPD | IPMC | (−) | (+) Intraductal | |
| 77/F | Body/tail | NS | 25 mm | None | DP | IPMA | (+) Focally | (+) Intraductal | |
| 71/M | Head/tail | NS | 40 mm | None | TP | IV | (+) Into duodenal and jejunal | (+) Intraductal | |
| Present case | 74/F | Head | 5.5 | None | AP | PPPD | Low-grade dysplasia | (+) | (+) Intra-abdominal bleeding |
RP: retroperitoneal; NS: not stated; AP: abdominal pain; PPPD: pylorus-preserving pancreaticoduodenectomy; AF: abdominal fullness; LOA: loss of appetite; DP: distal pancreatectomy; IV: invasive carcinoma; IPMA: intraductal papillary mucinous adenoma; IPMC: intraductal papillary mucinous carcinoma; TP: total pancreatectomy.
In many cases in the literature review, the tumors were malignant, and pancreatic duct dilation was seen. This may be accounted for by the fact that the tumor characteristics may change over time.
Some of the causative factors for intraductal bleeding in IPMN have been considered. Stasis of pancreatic flow of mucinous materials in the dilated pancreatic ducts might place high-pressure stress on the tumor, and this may play an important role in denuding the epithelium.1 Kurihara et al.5 described several processes, including potential fistula formation due solely to mechanical pressure from expansion by the tumor and/or thick mucus, accompanied by stromal invasion of the cancer and rupture through mucinous lakes within the stroma. In our patient, the cause of IPMN rupture and massive bleeding was not clear. Pancreatic pseudocysts sometimes develop intracystic bleeding, and there is a possibility that bleeding occurred into the IPMN cavity and formed a hematoma in our case. However, the histopathology of the surgical specimen showed epithelial lining within the cyst, and our patient had no history of acute pancreatitis or external injuries. For this reason, we believe that our case represented IPMN-associated hemorrhage.
The possible mechanism underlying IPMN rupture with intra-abdominal hemorrhage was as follows. The pancreatic cyst wall was lined by neoplastic cells. Bleeding of the neoplastic cell lining might have repeatedly occurred in the cyst, leading to breakdown and neovascularization. Mucinous materials and hematomas in the pancreatic cyst might have placed high-pressure stress upon the tumor epithelium. As a result, spontaneous rupture might have occurred.
Spontaneous IPMN rupture and bleeding are infrequent, but may be life-threatening. Even in patients with non-malignant IPMNs, follow-up examination may be needed if the size of the tumor was greater than 20 mm, or duct dilation was greater than 10 mm. We surmised that early preoperative diagnosis was important for effective management.
Conclusion
We reported a patient with pancreatic IPMN, resulting in massive and life-threatening bleeding. The findings from our case and a literature review indicated that pancreatic IPMN may carry a risk of spontaneous rupture with bleeding. Follow-up examinations are recommended even for IPMN patients without malignant findings because of the potential risk of rupture and bleeding with intra-abdominal hemorrhage. Clinicians should be aware of this possibility to ensure that patients are appropriately treated.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: All procedures performed in this case report involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
References
- 1. Yamada Y, Mori H, Hijiya N, et al. Intraductal papillary mucinous neoplasms of the pancreas complicated with intraductal hemorrhage, perforation, and fistula formation: CT and MR imaging findings with pathologic correlation. Abdom Imaging 2012; 37(1): 100–109. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas 2004; 28: 282–288. [DOI] [PubMed] [Google Scholar]
- 3. Farrell JL, Brugge WR. Intraductal papillary mucinous tumor of the pancreas. Gastrointest Endosc 2002; 5: 701–714. [DOI] [PubMed] [Google Scholar]
- 4. Jung IS, Shim CS, Cheon YK, et al. Invasive intraductal papillary mucinous tumor of the pancreas with simultaneous invasion of the stomach and duodenum. Endoscopy 2004; 36(2): 186–189. [DOI] [PubMed] [Google Scholar]
- 5. Kurihara K, Nagai H, Kasahara K, et al. Biliopancreatic fistula associated with intraductal papillary-mucinous pancreatic cancer: institutional experience and review of the literature. Hepatogastroenterology 2000; 47: 1164–1167. [PubMed] [Google Scholar]
- 6. Lee SE, Jang JY, Yang SH, et al. Intraductal papillary mucinous carcinoma with atypical manifestations: report of two cases. World J Gastroenterol 2007; 13: 1622–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol 2010; 45(10): 1080–1089. [DOI] [PubMed] [Google Scholar]
- 8. Koizumi M, Sata N, Yoshizawa K, et al. Post-ERCP pancreatogastric fistula associated with an intraductal papillary-mucinous neoplasm of the pancreas—a case report and literature review. World J Surg Oncol 2005; 19(3): 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oku T, Maeda M, Wada Y, et al. Intraductal oncocytic papillary neoplasm having clinical characteristics of mucinous cystic neoplasm and a benign histology. JOP 2007; 8(2): 206–213. [PubMed] [Google Scholar]
- 10. Zanelli M, Casadei R, Santini D, et al. Pseudomyxoma peritonei associated with intraductal papillary-mucinous neoplasm of the pancreas. Pancreas 1998; 17: 100–102. [DOI] [PubMed] [Google Scholar]
- 11. Mizuta Y, Akazawa Y, Shiozawa K, et al. Pseudomyxoma peritonei accompanied by intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2005; 5: 470–474. [DOI] [PubMed] [Google Scholar]
- 12. Imaoka H, Yamao K, Salem AA, et al. Pseudomyxoma peritonei caused by acute pancreatitis in intraductal papillary mucinous carcinoma of the pancreas. Pancreas 2006; 32: 223–224. [DOI] [PubMed] [Google Scholar]
- 13. Nepka CH, Potamianos S, Karadana M, et al. Ascitic fluid cytology in a rare case of pseudomyxoma peritonei originating from intraductal papillary mucinous neoplasm of the pancreas. Cytopathology 2009; 20: 271–273. [DOI] [PubMed] [Google Scholar]
- 14. Rosenberger LH, Stein LH, Witkiewicz AK, et al. Intraductal papillary mucinous neoplasm (IPMN) with extra-pancreatic mucin: a case series and review of the literature. J Gastrointest Surg 2012; 16(4): 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagano H, Koneri K, Honda K, et al. Biliopancreatic fistula and abscess formation in the bursa omentalis associated with intraductal papillary mucinous cancer of the pancreas. Int J Clin Oncol 2009; 14: 460–464. [DOI] [PubMed] [Google Scholar]