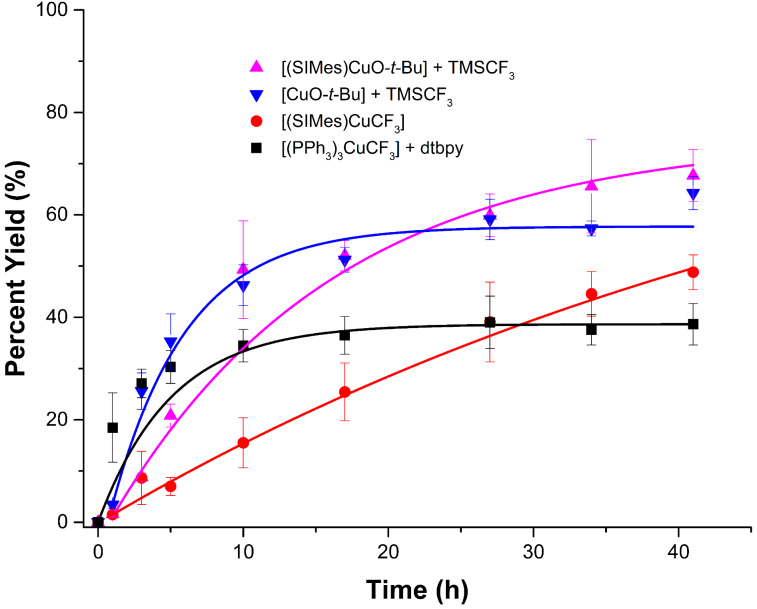
Figure 2.
Yields of 4-(trifluoromethyl)-1,1’-biphenyl over time for the systems described in Scheme 1. Conditions for C1: [(PPh3)3Cu(CF3)] + dtbpy: toluene, 80 °C. Conditions for A1: [(SIMes)Cu(CF3)] and A2 ([(SIMes)Cu(O-t-Bu)] + TMSCF3: DMI/benzene (1.5:7.5), 50 °C. DMI = 1,3-dimethyl-2-imidazolidinone. Conditions for B2: [(Cu(O-t-Bu)]4 + phen +TMSCF3: DMF, 50 °C. Yields were monitored by gas chromatography relative to a calibrated internal standard.