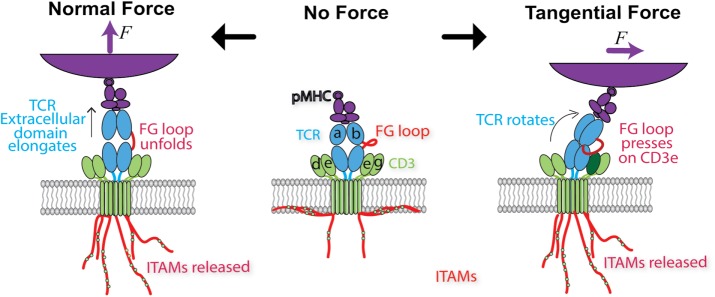
FIGURE 5:
Models of how force may trigger TCR signaling. Middle, schematic of the ligated, unloaded, and untriggered TCR. Soluble pMHC binds to the TCR V domains, while the CTs of the TCR-associated CD3 chains remain buried in the lower leaflet of the cell membrane, preventing ITAM phosphorylation. Left, a force normal to the cell membrane pulls on the TCR, extending the length of the complex by ∼10 nm. While the structural region responsible for such conformational change has not been identified, here the FG loop connecting the Cβ and Vβ domains is assumed to unfold to result in an extended conformer and in catch-bond formation. Force propagated across the TCR-CD3 connection is assumed to release the CD3 CTs for phosphorylation of the ITAMs. Right, when a force tangential to the cell surface is applied to the ligand binding site of the TCR that also experiences a lateral reaction force from its membrane anchor, a torque is generated to rotate the complex, which is assumed to allow the FG loop to press down on the CD3ε ectodomain to expose the cytoplasmic ITAMs in a piston-like manner.