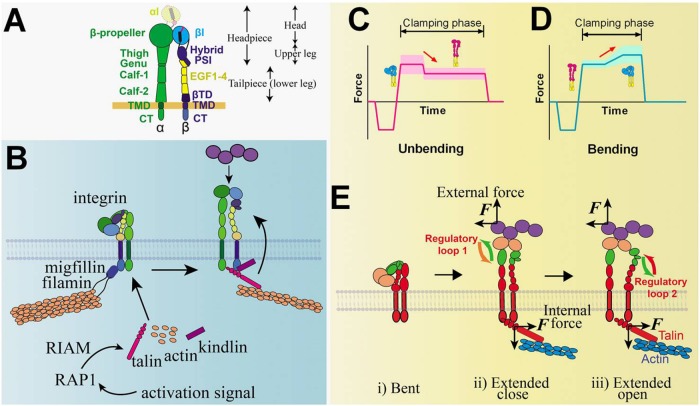
FIGURE 6:
Integrin structure and signaling models and illustrative BFP signals that detect integrin bending and unbending. (A) Integrin structure. Domains of the integrin and the ranges of integrin head, upper leg, headpiece, and tailpiece (or lower leg) are annotated. αI domain that exists only in αI-bearing integrins is distinguished by a lighter color and dashed lines. (B) A model of integrin inside-out signaling mediated by RAP1 and RIAM. (C, D) Illustrative BFP force vs. time traces depicting respective integrin unbending (C) and bending (D) conformational change events, denoted by red arrows. The conformations of the integrin are inserted accordingly, with red and blue indicating the extended and bent conformers of the ectodomain, respectively. Widths of the shaded regions reflect the thermal fluctuation amplitude of each trace in the clamping phase. (E) Postulated model of integrin-mediated mechanosensing without prior talin engagement. External force pulls on the i) bent integrin headpiece and allosterically induces ii) ectodomain extension, iii) hybrid domain swing-out, and αβ-subunit separation below the headpiece due to an endogenous lateral force pulling on the βCT. The αβ separation at the CT presumably recruits intracellular molecules and initiates downstream signaling. The ligand, integrin domains, and talin are colored based on their respective roles in the four-step mechanosensing model (cf. Figure 1). Proposed regulatory loops 1 (between mechanoreception and mechanotransmission) and 2 (between mechanotransmission and mechanotransduction) are denoted.