TABLE 1:
Parameters of the mathematical model of the S. pombe cytokinetic ring.
| Symbol | Meaning | Value | Legend |
|---|---|---|---|
| ρ0 | Steady-state total density of nodes at onset of constriction | 16.1 μm−1 | (A) |
| l | Length of F-actin per node at the onset of constriction | 2.7 μm | (A) |
| fmyo | Force exerted by one Myo2 head | 1.11 ± 0.43 pN | (B) |
| fnode | Force exerted by one node on one filament that passes through it | 0.41 ± 0.16 pN | (B) |
| γanc | Membrane drag coefficient of the node anchor | 810 ± 370 pN s μm−1 | (B) |
| τturn | Turnover time of nodes | 18.6 s | (C) |
| L0 | Initial length of the ring | 11.8 μm | (D) |
| frep | Repulsive force between nodes | 0.1 pN | (E) |
| brep | Range of node repulsive force | 0.1 μm | (E) |
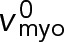 |
Load-free velocity of myosin Myo2 | 240 nm s−1 | (F) |
| - | Ring tension | 391 ± 154 pN | (G) |
| - | Myosin-II Myo2 node velocity | 22 ± 10 nm s−1 | (H) |
Errors are SDs for experimentally measured values and calculated parameters.
(A) Calculated from the experiments of Pelham and Chang (2002), Wu and Pollard (2005), Courtemanche et al. (2016), and Laplante et al. (2016).
(B) Obtained in this study. Associated error is due to uncertainty in prior experimental measurements of node velocity and ring tension.
(C) Obtained from FRAP experiments on YFP-Myo2 in constricting rings as measured in Sladewski et al. (2009).
(D) Previously measured using fluorescence microscopy on GFP-Cdc4 (Pelham and Chang, 2002).
(E) Chosen such that the final mean cluster width after aggregation was ∼150 nm (Materials and Methods).
(F) Obtained from gliding filament assays of Stark et al. (2010) as described in Supplemental Materials and Methods.
(G) Obtained from tension measurement experiments in Stachowiak et al. (2014).
(H) Obtained from FPALM myosin Myo2 node velocity measurements in Laplante et al. (2016).
