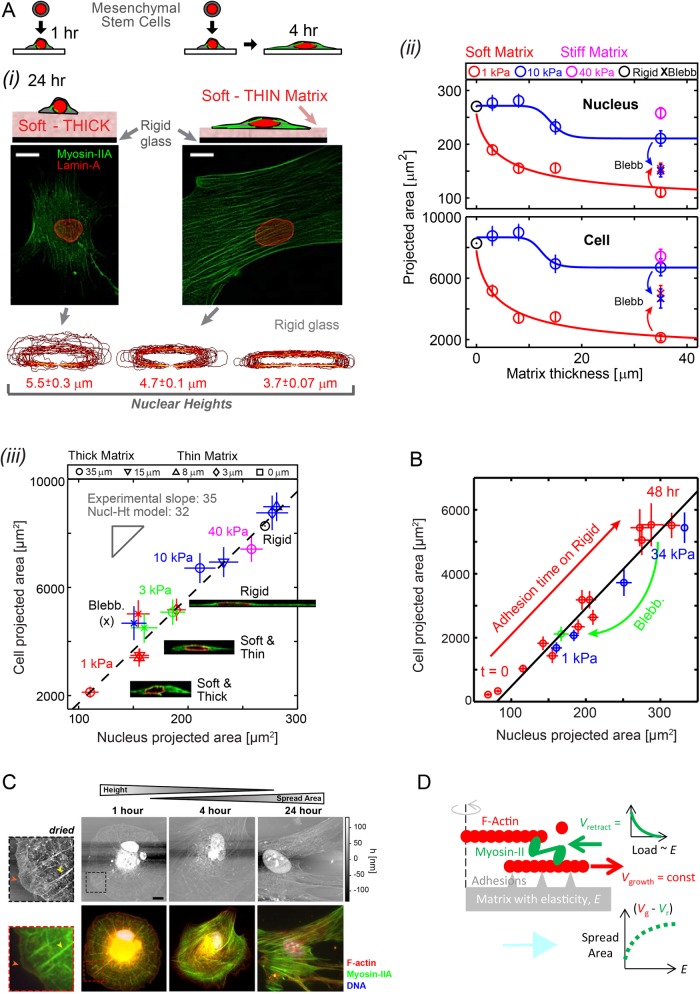
FIGURE 1:
Cells feel many microns into soft microenvironments. (A) (i) Mesenchymal stem cells (MSCs) and their nuclei are spread more on gels that are soft-and-thin (1 kPa; 2–3 μm) compared to soft-and-thick (∼35 μm) at 24–36 h in culture (scale bars = 10 μm). Bottom, x-z contours of lamina from confocal stacks of immunostained lamin-A,C. Nuclear height (average ± SEM; n > 25) is maximal on thick and soft gels but nuclei become increasingly flattened on thin-and-soft gels (p = 0.006) and rigid glass (p < 0.001). (ii) Mean projected areas of nuclei and cells vs. matrix thickness. Hill function exponents are α = 0.8 and 15 for 1 and 10 kPa gels, respectively. Tactile length scales are defined as the thickness below which cells or nuclei spread more than a measurable 10% relative to cells on thick gels of the same E: ht ≈ 25 μm for 1 kPa, ht ≈ 15 μm for 10 kPa, and ht ≈ 0 μm for 40 kPa, making the latter indistinguishable from collagen-coated glass (i.e., “rigid”). Blebbistatin (Blebb) inhibits myosin-II and eliminates spreading differences on different matrices. (iii) Linearity of cell vs. nucleus projected area is maintained across matrices of different elasticities and thicknesses and is also satisfied by Blebb-treated myosin-inhibited cells. Inset images of x-z cross sections show spread cell height is constrained by nuclear height. (B) Cell vs. nuclear spreading kinetics on rigid glass (red) tracks the “steady-state” projected area of cells on diverse gels (blue) or with myosin-II inhibition by Blebb (green). (C) The dynamics of cell adhesion and spreading were interrogated by AFM (top) and immunostaining (bottom) to show organization of protein of interests with z-axis topography. MSCs were cultured in a standard cell-culture plastic plate and fixed at times ranging between 1 h and 1 d. To facilitate AFM imaging, cells were lipid-membrane stripped and air dried before immunostaining. With adhesion time, cells increasingly spread, and nuclei stretch and flatten down against the substrate and prominent stress fibers developed, consistent with increased generation of contractile forces. At 1 h, cells maintain a spherical morphology and stress fibers are radially distributed (yellow arrowheads, zoom-in images) with a wide lamellipodium structure (red arrowheads) but after 4 h of adhesion symmetry is broken and within 24 h cells obtain a typical mesenchymal-like morphology typical of stiff matrices. Unlike the immunofluorescence (IF) images, AFM micrographs are in scale (scale bar: 10 µm). z-Axis heights measured by AFM is an underestimate, due to fixing, membrane-stripping, and dehydration of the cells. (D) A simple Hill-type model of cell spreading in which myosin-II rectifies F-actin polymerization depending on the resistance provided by matrix elasticity.