Table 1.
Dehydrative cyclization scope.[a]
| Entry | Substrate | Product | Yield[b] | dr[c] |
|---|---|---|---|---|
| 1 |
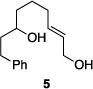
|
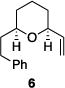
|
81% | 1.7:1 |
| 2 |
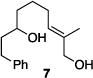
|
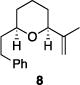
|
81% | 2.6:1 |
| 3 |
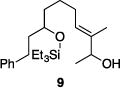
|

|
71% | >30:1 |
| 4 |
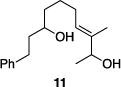
|
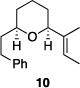
|
86% | >30:1 |
| 5 |
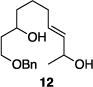
|
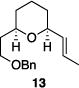
|
86% | 7.6:1 |
| 6 |
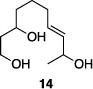
|
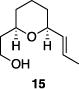
|
85% | >15:1 |
| 7 |
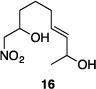
|
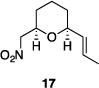
|
84% | 16:1 |
| 8 |
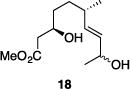
|
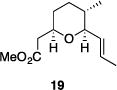
|
77% | >30:1 |
| 9 |
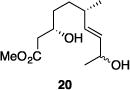
|
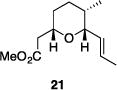
|
56% | 2.9:1 |
See the Supporting Information for experimental procedures and spectral data.
Combined yield of the diastereomeric mixture.
Determined by 1H NMR analysis of the crude product mixture.
