Abstract
Background
Before the introduction of highly active antiretroviral therapy (HAART), cardiac mortality and morbidity were common in HIV-infected children.
Objective
To identify long-term cardiovascular effects of HAART in HIV-infected children.
Methods
The HAART Associated Cardiotoxicity in HIV-Infected Children (CHAART-2) study prospectively compared 148 echocardiograms from 74 HAART-exposed children to 860 echocardiograms from 140 HAART-unexposed but HIV-infected children from the Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) study. Both studies used similar protocol, centralized echocardiographic interpretation and measures expressed as Z-scores referenced to healthy controls. Associations between HAART exposure and echocardiographic measures were evaluated using generalized estimating equations.
Results
Comparing the HAART exposed and HAART unexposed groups, any HAART exposure was positively associated with left ventricular (LV) fractional shortening (Z-score for difference = 1.07; p = 0.02) and HAART exposure duration (Z-score difference per year = 0.17; p = 0.003. LV mass was negatively associated with any HAART exposure (Z-score difference = −0.64; p = 0.01) as was septal thickness (Z-score difference = −0.93; p = 0.001). Duration of HAART exposure was negatively associated with LV end-systolic dimension and heart rate (Z-score difference per year= −0.11; p = 0.05 and Z-score difference per year= −0.10; p = 0.002, respectively). During 11 years of follow-up, in the HAART-exposed group, LV mass and LV end-diastolic septal thickness were lower while LV contractility and LV fractional shortening were higher when compared to the HAART-unexposed group.
Conclusions
Cardiac structure and function were better in perinatally HIV-infected children exposed to HAART than in those of similar children from the pre-HAART era but did decline over time. Evidence-based strategies for cardiovascular monitoring are needed to inform treatment decisions to improve long-term cardiovascular health.
Keywords: antiretroviral therapy, cardiomyopathy, HIV, pediatric
Introduction
Before the widespread use of highly active antiretroviral therapy (HAART) in children in the late 1990s, children infected with HIV commonly experienced serious cardiac morbidity and mortality. Observed abnormalities of left ventricular (LV) structure and function were strong predictors of mortality in this patient group (1,2). Specifically, the observed abnormalities included dilated cardiomyopathy, abnormal LV contractility, aortic vasculopathy, and heart failure (1–9). Now, HIV-infected children experience a lifelong exposure to HAART.
Additionally, most of these children were exposed in utero to combination antiretroviral therapy (ART), including HAART. The cardiac implications of concomitant exposure to HIV and ART both prenatally and postnatally are unknown.
In the pre-HAART era, the most comprehensive study of the cardiac effects in HIV-infected children was the NHLBI-funded Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) study, which was conducted from 1990 to 1997 (2). This study found that subclinical decreased LV contractility and increased LV mass predicted death in HIV-infected children (1–5). These abnormal echocardiographic findings were also consistent with postmortem diagnoses of cardiomegaly in these children (3). The P2C2 HIV study also reported that HIV-infected children more than 5 years old were more likely to die of cardiac causes than from pulmonary or other infections (10).
The cross-sectional NIH-funded multicenter Pediatric HIV/AIDS Cohort Study (PHACS) found that HIV-infected children in the HAART era had significantly more normal echocardiographic findings (LV contractility, LV fractional shortening, LV end-systolic dimension) than did the HAART unexposed historical controls from the P2C2 HIV study. The same was true when comparing echocardiographic parameters between a separate HIV-exposed but uninfected group from PHACS and the HIV-infected children in the P2C2 HIV study (11).
The authors concluded from this cross-sectional study that HAART exposure was generally cardioprotective, but longitudinal studies were needed to confirm this conclusion. To determine the longitudinal cardiac effects of ART and chronic HIV exposure in these children, we compared the echocardiographic results from the prospective multicenter NHLBI-funded HAART Associated Cardiotoxicity in HIV-Infected Children (CHAART-2) cohort study to those of historical controls from P2C2 HIV study conducted in the pre-HAART era.
Methods
Study Population
The CHAART-2 study prospectively identified 74 HIV-infected children 3–16 years old who had been exposed to combination ART, including HAART, between November 2004 and November 2007 in the Women and Infant Transmission Study (WITS) and had been enrolled at one of the 7 CHAART 2 study sites, 6 of which also participated in the P2C2 HIV study (12). HAART is a specific type of combination ART which consists of a combination of 3 or more antiretroviral drugs from at least 2 drug classes: nucleoside reverse transcriptase inhibitors; nonnucleoside reverse transcriptase inhibitors; or protease inhibitors. The CHAART-2 participants also included 14 HIV-infected children, 3–16 years old, who were exposed to combination ART/HAART and enrolled at the non-WITS study site. Since at the time of their study echocardiogram, 91% of CHAART-2 participants were reported as HAART-exposed, hereafter, the CHAART-2 participants will be referred to as the HAART-exposed cohort. Maternal exclusion criteria for CHAART-2 participants included diabetes, phenylketonuria, a Mendelian or chromosomal defect, a heart defect requiring medication or surgery, or pregnancy exposures to chemotherapy, radiation, or drugs associated with heart disease in their children. Other exclusion criteria included children under 2 years of age, and children whose maternal ethnicity was other than black, white, or Hispanic.
The P2C2 HIV study prospectively described cardiovascular disorders in HIV-infected children born to HIV-infected mothers enrolled between 1990 and 1994 at 5 clinical centers (13). The 140 HIV-infected children unexposed to combination ART/HAART in that study constituted the HAART-unexposed control group of the current study. Some of these children eventually received zidovudine, but not HAART. Henceforth, the P2C2 HIV study participants will be referred to as the HAART-unexposed cohort.
The CHAART-2 and P2C2 HIV sites received Institutional Review Board approval to collect the serial echocardiograms from identified patients. Written and informed consent was obtained from parents or guardians for all patients.
Nadir CD4% and most recent HIV viral load values were obtained from each participant in both cohorts. Combination ART was defined as the use of 2 or more ART drugs without a non-nucleoside reverse transcriptase inhibitor or protease inhibitor, and HAART was defined as the use of 3 or more ART drugs, including a non-nucleoside reverse transcriptase inhibitor or a protease inhibitor.
Study Echocardiograms
In the HAART-exposed cohort, LV function was evaluated with serial echocardiograms performed every 6 months. In the HAART-unexposed cohort, echocardiograms were performed every 4 to 6 months. These studies provided both LV functional and structural data. In this report, we analyze echocardiographic data for up to 9 years of HAART exposure.
The P2C2 HIV echocardiographic acquisition protocol was followed (2, 14) without specific mention of evaluation of cardiac disease. CHAART 2 echocardiographic data were digitized by the same independent, blinded cardiologist who had measured the prior P2C2 HIV echocardiograms and the healthy control group from the Boston Children’s Hospital, which were used to calculate the normative echocardiographic parameter Z-scores described below (15). Z-scores represent the numer of standard deviations from the population mean and are used in pediatric cardiology to account for difference in age and body size. A Z-score of + or − 2 is usually considered abnormal.
Statistical Methods
All effects estimated in this report were calculated using the Generalized Estimating Equation (GEE) model in SAS. A normal distribution assumption was used in the model with an identity link function. A repeated measures effect was included for subjects in each model and an exchangeable correlation structure was used to model the repeated measures. There were approximately 200 subjects represented in each model: on average, 60% of these subjects had complete data for each analysis. Fixed effects were included for: the intercept, gender, race/ethnic background, HAART treatment, the subject’s age, the number of years a subject was exposed to HAART treatment, the subject’s CD4 percent, and the log base 10 of the subject’s HIV viral load. Statistical tests were performed by using the Wald estimates obtained from the empirical model estimate and its standard error (16). To adjust for differences in age and body size, echocardiographic measurements were expressed as Z-scores from a distribution of similar measurements of healthy children with normal echocardiograms from the Boston Children’s Hospital (the Boston control group) (17). Z-scores of zero correspond to the average value of this normative control group, with negative scores indicating below-average values and positive scores to above-average values. Z-scores are units of standard deviation, similar to those used in growth charts for age versus height and weight, which are provided by the Centers for Disease Control and Prevention. As long as the populations being compared do not differ markedly from healthy controls in factors such as body mass index, level of habitual exercise and blood pressure, in the absence of cardiac effects from HIV/HAART exposures, the mean Z-scores of the treatment cohorts should approximate those of healthy controls.
Each model was adjusted for sex, maternal ethnicity, age at examination, nadir CD4%, and the most recent measurement of HIV viral load. The effect of HAART was analyzed with two covariates in each model, one as the total duration of HAART exposure and the other as any HAART exposure at any time during the study period. Alpha was set at 0.05. Data were analyzed with the 9.4 statistical software program.
Results
The median (range) age at the most recent echocardiographic follow-up was 149 months (49–201 months) for the HAART-exposed cohort (n = 74) and 73 months (41–201 months) in the HAART-unexposed cohort (n = 140). Infant and maternal characteristics were generally similar between the cohorts (Table 1). The HAART-unexposed children had lower nadir CD4% and higher HIV viral loads at most recent follow-up (both p values < 0.001). All children in the CHAART cohort were exposed to combination ART; 91% were exposed to HAART and 9% to other combination ART regimens. In the HAART-unexposed cohort, 91% were exposed to zidovudine monotherapy and 9% were ART unexposed.
Table 1.
Demographic Characteristics of 214 HIV-infected Children by HAART exposure.
| Variable | HAART- exposed children (n = 74) |
HAART-unexposed children (n = 140) |
p value* |
|---|---|---|---|
| Characteristics of mothers during pregnancy – no. (%) | |||
| Ethnicity | 0.39 | ||
| Black | 39 (53) | 60 (43) | |
| White | 9 (12) | 21 (15) | |
| Hispanic | 26 (35) | 59 (42) | |
| Age at delivery, years | 0.08 | ||
| < 30 | 39 (53) | 82 (59) | |
| ≥ 30 | 34 (46) | 42 (30) | |
| Unknown | 1 (1) | 16 (11) | |
| Tobacco use | 0.87 | ||
| Yes | 30 (41) | 57 (41) | |
| No | 34 (46) | 68 (49) | |
| Unknown | 10 (14) | 15 (11) | |
| Alcohol use | 0.006 | ||
| Yes | 28 (38) | 29 (21) | |
| No | 36 (49) | 92 (66) | |
| Unknown | 10 (14) | 19 (14) | |
| Illicit drug use | 0.24 | ||
| Yes | 27 (36) | 45 (32) | |
| No | 33 (45) | 80 (57) | |
| Unknown | 14 (19) | 15 (11) | |
| Characteristics of children, n (%) | |||
| Sex | 0.85 | ||
| Male | 36 (49) | 70 (50) | |
| Female | 38 (51) | 70 (50) | |
| Premature birth (<37 week gestational age) | 0.66 | ||
| Yes | 19 (26) | 31 (22) | |
| No | 49 (66) | 97 (69) | |
| Unknown | 6 (8) | 12 (9) | |
| Birth weight | 0.82 | ||
| < 2500 grams | 18 (24) | 34 (24) | |
| ≥ 2500 grams | 44 (60) | 90 (64) | |
| Unknown | 12 (16) | 16 (11) | |
| Lowest CD4% (95% CI) | 17.7 (15.5–19.2) 12.0 (10.3–13.8) | <0.001 | |
| Last known number of log10- transformed HIV RNA copies (95% CI) | 1.43 (0.93–1.93) | 4.37 (4.21–4.53) | <0.001 |
| Maximum ART exposure status (%) | |||
| None | 0 (0) | 78 (9) | – |
| Zidovudine Monotherapy | 0 (0) | 782 (91) | – |
| Combination ART | 13 (9) | 0 (0) | – |
| HAART | 135 (91) | 0 (0) | – |
| Echocardiography age groups (%)† | |||
| 3 – 4 years | 11 (7) | 322 (37) | – |
| 5 – 6 years | 10 (7) | 260 (30) | – |
| 7 – 8 years | 12 (8) | 126 (15) | – |
| 9 – 10 years | 18 (12) | 91 (11) | – |
| 11 – 12 years | 51 (34) | 35 (4) | – |
| 13 – 14 years | 37 (25) | 19 (2) | – |
| 15 – 16 years | 9 (6) | 7 (1) | – |
ART=antiretroviral therapy; HAART=highly active antiretroviral therapy
Chi-square analysis: Observations with unknown data were excluded from the p value calculation.
The GEE analysis included 148 echocardiograms from the HAART-exposed cohort and 860 echocardiograms from the HAART-unexposed cohort. Both functional and structural variables differed between the cohorts (Table 2). Mean LV fractional shortening and LV contractility were significantly and positively associated with both overall HAART exposure as well as HAART exposure duration (LV fractional shortening: p = 0.02, 0.003 and LV contractility: 0.002, 0.02, respectively).
Table 2.
Cardiac Parameters among 214 HIV-infected Children Born to HIV-infected Mothers, by HAART Exposure Status
| Unadjusted Population Means | GEE Parameter Estimate* | |||
|---|---|---|---|---|
|
|
||||
| Parameter Z-score | HAART Exposed | HAART Unexposed | Effect of Any HAART Exposure Overall Difference | Effect of Length of Cumulative HAART Exposure Difference Per Year |
| LV fractional shortening | 0.26 | −1.19 | 1.07, p = 0.02 | 0.17, p = 0.003 |
| LV contractility | 0.09 | −0.88 | 1.25, p = 0.002 | 0.13, p = 0.02 |
| LV end-systolic afterload | −1.09 | 0.54 | −0.50, p = 0.18 | −0.05, p = 0.30 |
| Systolic blood pressure | −0.14 | −0.36 | 0.61, p = 0.04 | −0.02, p = 0.66 |
| LV mass† | −0.25 | 0.53 | −0.64, p = 0.01 | 0.01, p = 0.72 |
| LV end-systolic dimension† | −0.12 | 0.98 | −0.67, p = 0.13 | −0.11, p = 0.05 |
| End-diastolic septal wall thickness† | −0.45 | 0.41 | −0.93, p = 0.001 | 0.05, p = 0.13 |
| LV end-diastolic posterior wall thickness† | 0.04 | −0.20 | −0.03, p = 0.91 | 0.004, p = 0.91 |
| Heart rate† | 0.07 | 0.80 | 0.22, p = 0.33 | −0.10, p = 0.002 |
HAART = highly active antiretroviral therapy; GEE = generalized estimating equation; ART = antiretroviral therapy; LV = left ventricular.
Estimates represent the difference in population means between the HAART exposed (comparison) and HAART unexposed (reference) groups adjusted for age, sex, ethnicity, lowest CD4%, and the last known HIV RNA copy number.
Lowest CD4% is a significant factor with p < 0.05. In no instance was log HIV RNA statistically significantly related to any cardiac outcome variable.
LV afterload was not associated with either any HAART exposure or HAART exposure duration. Systolic blood pressure was associated with HAART exposure (p = 0.04) but not with the duration of exposure. Left ventricular mass was negatively associated with HAAART exposure (p = 0.01), as was end-diastolic septal thickness (p = 0.001), but not with HAART exposure duration. Heart rate was negatively associated with the duration of HAART exposure.
We also compared the echocardiographic Z-score parameters between the HAART-exposed and unexposed cohorts over the follow-up period (Figure 1). Both LV fractional shortening and LV contractility were consistently higher in the HAART-exposed cohort (Figures 1a–1b; p = 0.017 and 0.002, respectively). In the HAART-exposed group, LV contractility did decrease with increasing follow-up so that the mean value at 11 years was lower than at baseline (p = 0.05) and was similar to the baseline value for the HAART-unexposed cohort. LV mass and LV end-diastolic septal thickness were also lower in the HAART-exposed cohort over the follow-up period (Figures 1E and 1G; p = 0.0135 and < 0.001, respectively).
Figure 1. A comparison of longitudinal cardiac measurements of 74 HAART-exposed children from the CHAART-2 study and 140 HAART-unexposed children from the P2C2 HIV study cohort.
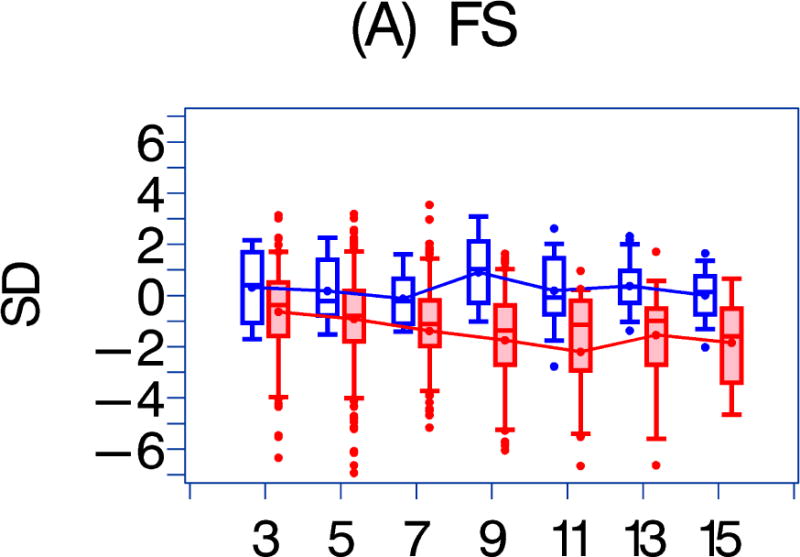
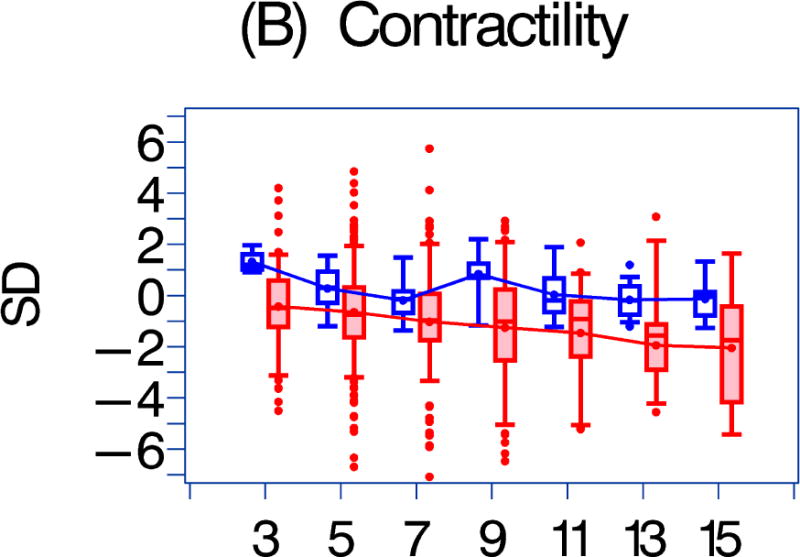
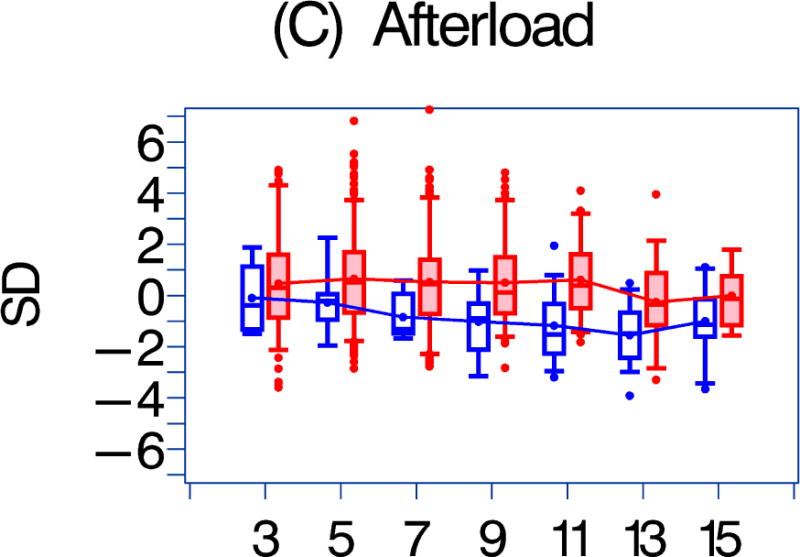
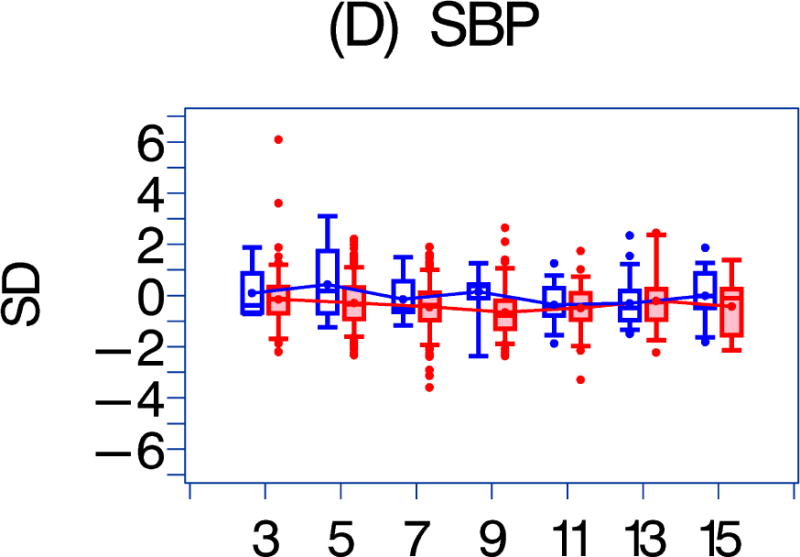
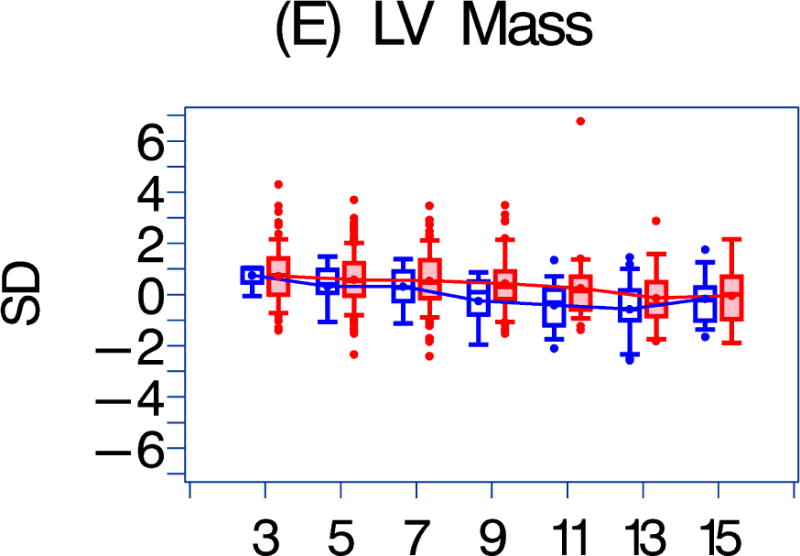
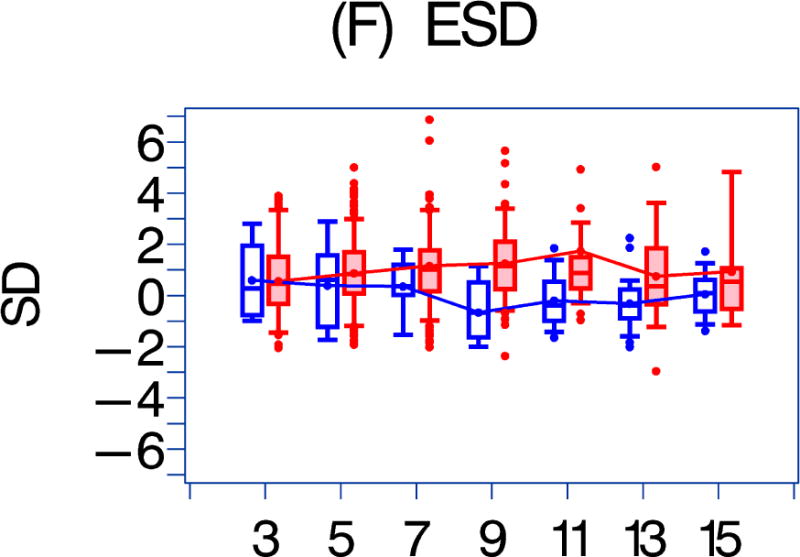
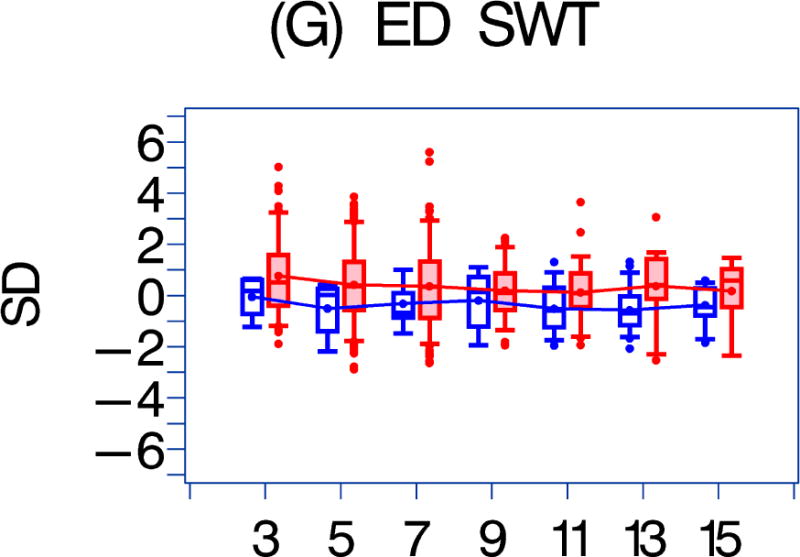
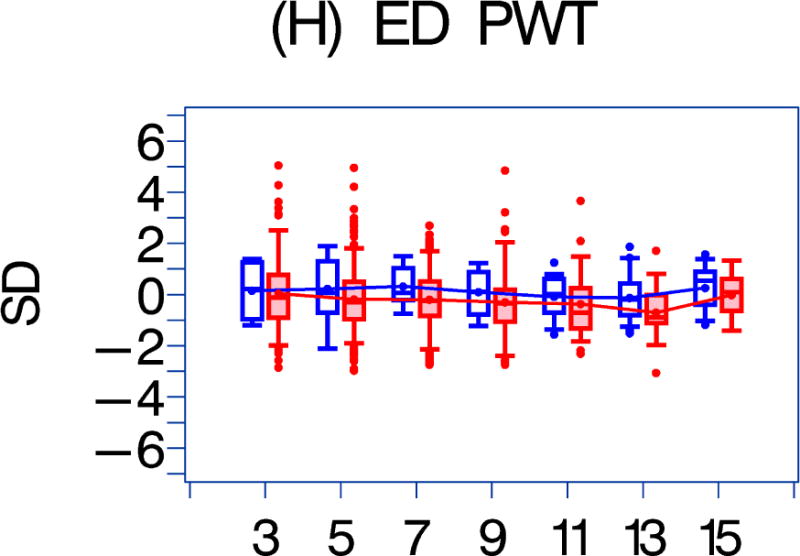
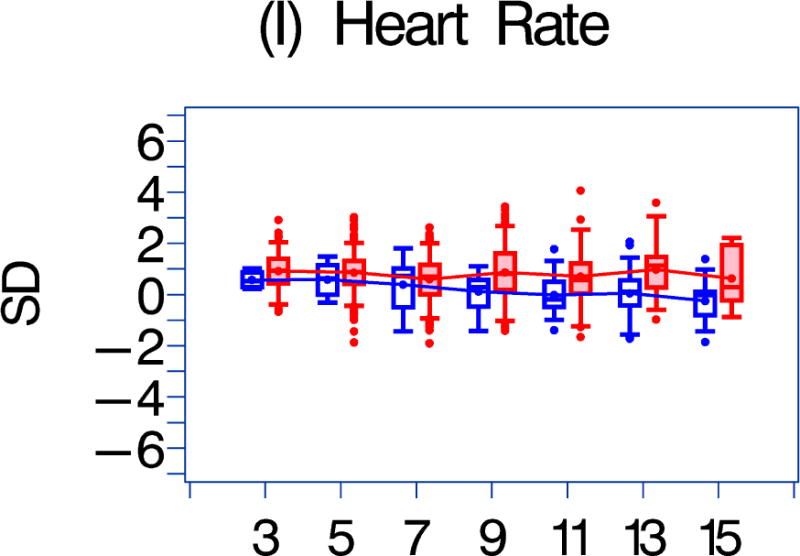
Using GEE adjusted for cohort, gender, race-ethnicity, age, duration of HAART exposure, nadir CD4%, and most recent HIV viral load (HIV RNA copies/ml), echocardiographic parameters were compared between HAART-unexposed (RED) and HAART-exposed (BLUE) groups: The X-axis is years of follow-up. The Y-axis is number of standard deviations from the mean (Z-score). The p values represent interaction between HAART exposure status and years of follow-up. 1A. LV fractional shortening Z-score. (p = 0.0167); 1B. LV contractility (LV stress velocity index) Z-score. (p = 0.0023); 1C. LV afterload (LV end-systolic stress) Z-score. (p = 0.1844); 1D. Systolic blood pressure Z-score. (p = 0.0435); 1E. LV mass Z-score. (p = 0.0135); 1F. LV end-systolic dimension Z-score. (p = 0.1258); 1G. End-diastolic septal wall thickness Z-score. (p < 0.0013); 1H. LV end-diastolic posterior wall thickness Z-score (p = 0.9091); 1I. HAART-unexposed versus HAART-exposed heart rate Z-score (p = 0.3321).
Discussion
We compared echocardiographic measurements of LV structure and function in a historical cohort of HIV-infected children from the pre-HAART era (the P2C2 HIV study) to those in a more recent HIV-infected cohort who were exposed to HAART (the CHAART-2 study) (Central Illustration). In the HAART-exposed cohort, cardiovascular structural and functional characteristics changed in varying degrees over time. Specific echocardiographic characteristics were also associated with HAART-exposure or the duration of HAART-exposure.
Central Illustration. Highly Active Antiretroviral Therapy-associated Cardiac Changes in HIV-infected Children.
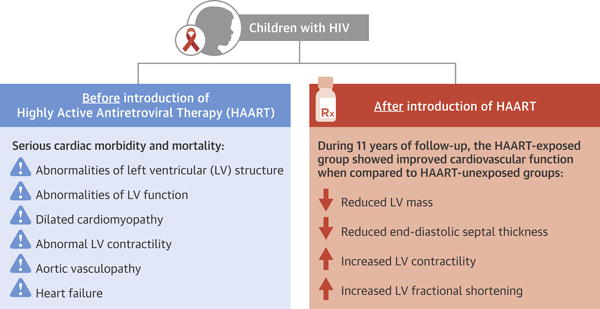
A comparison of longitudinal cardiac measurements of 74 HAART-exposed children from the CHAART-2 study and 140 HAART-unexposed children from the P2C2 HIV study cohort. During 11 years of follow-up, perinatally HIV-infected children exposed to highly active antiretroviral therapy (HAART) had consistently higher left ventricular (LV) fractional shortening and contractility while LV mass and end-diastolic septal thickness (ED SWT) were consistently lower when compared to a HAART-unexposed group.
Left ventricular fractional shortening and LV contractility were higher in HAART-exposed children, and the difference became more pronounced over time. A 4% decrease in LV fractional shortening can greatly increase mortality (1); therefore, maintaining LV fractional shortening is an important marker of cardiac preservation. Our findings support the conclusion that HAART use in HIV-infected children appears to be generally cardioprotective, findings consistent with those in HAART-exposed, perinatally HIV-infected children from the more recent PHACS (11). However, the consistent decrease in LV contractility with increasing followup in the HAART-exposed group is of concern because LV contractility at 11 years was similar to that for HAART-unexposed children at initial assessment. Whether this downward trajectory of LV contractility will continue is unknown. This suggests that HAART therapy may delay by about a decade, but not prevent, the development of HIV-associated cardiomyopathy in this population.
Before the HAART era, children with vertically transmitted HIV had poor outcomes (18). Cardiomyopathy was among the top causes of morbidity and mortality. In a report from the pre-HAART era (1984–1994) published in the Journal of the American College of Cardiology in 2003, we reported that serious cardiac effects were common in perinatally HIV-infected children. Children at high risk for adverse cardiac outcomes included those with recurrent infections, wasting syndrome, encephalopathy, a low CD4%, and a younger age at diagnosis (6). In the HAART era, cardiomyopathy and heart failure have become increasingly rare in these children with intermediate follow-up, but long term follow-up studies are lacking to understand the lifetime risk of cardiovascular morbidity and mortality in this population (19, 20). Our findings of consistently more normal LV mass and septal thickness Z-scores and are consistent with the decreased prevalence of dilated cardiomyopathy in the HAART era.
A recent PHACS analysis compared HIV-infected children who received HAART, HIV-exposed but uninfected children, and many of the same historical HIV-infected but HAART-unexposed children enrolled in the current study (11). The results varied by the severity of HIV: children with the poorest predictive markers of HIV-related health status, such as low nadir CD4 counts and high HIV viral loads, had the poorest echocardiographic markers of LV function. Even when controlled for confounders, the significance of the relationship between poorer health status and worsened echocardiographic parameters persists.
Although the specifics of which medications are used and in what combination over time are important in determining their efficacy in treating HIV and in minimizing side effects, the data on traditional echocardiographic measurements, CD4, HIV viral load, and anthropometric characteristics presented here indicate that with longer follow-up children with HIV had significantly better measurements of cardiac and overall health when treated with HAART than without.
As HIV-infected children reach adolescence and beyond, early evaluation of traditional lifestyle risk factors for increased cardiovascular risk should be considered (21). Studies advocate for smoking cessation, blood pressure checks, and screening for and treatment of insulin resistance, lipid abnormalities, and hypercoagulable states in HIV-infected people (22). The age at which routine cardiovascular screening should begin in these children remains uncertain. This same uncertainty also applies to ongoing cardiovascular assessment, possibly including echocardiograms, as these children age into young adulthood.
Strengths and Limitations of the Study
This study compared well-characterized HIV-infected children followed longitudinally with very similar protocols, the same study endpoints, and all echocardiograms were measured centrally. Withholding HAART from HIV-infected patients is unethical, so we used historical controls from the pre HAART era. The HIV viral strains to which our historical cohort was exposed likely differs from that in the HAART era, and any effects of this difference are unknown.
Another potential limitation is that the children in this study were followed closely at large clinical centers with specially trained staffs. Differences in the quality of care could limit generalizing our findings to larger groups of HIV-infected children. Finally, with results from this population for follow-up only during childhood and adolescence and that subclinical LV dysfunction in children does not usually resolve examining both cohorts as they age into adulthood may be necessary to identify any longer-term effects of ongoing HAART exposure on LV structure and function in perinatally HIV-infected children.
Conclusions
We believe this is the first longitudinal study comparing cardiac status in HIV-infected children in the eras before and after the introduction of HAART. In the decade since the CHAART 2 study ended, although new individual antiretroviral drugs have been introduced, the composition of HAART regimens in terms of antiretroviral drug classes has not changed appreciably. Therefore, we believe that our findings are relevant to the care of perinatally HIV-infected children in the current era. Despite concerns about cardiovascular side effects, exposure to HAART in perinatally HIV-infected children was associated with better measurements of LV structure and function than those of children from the pre-HAART era. Cardiac function in HAART-exposed children did decline with increasing follow-up, indicating the need for prospective studies as these children become adults and are increasingly exposed to non-HAART-related cardiovascular risk factors. Such studies should result in evidence-based strategies regarding the type and frequency of cardiovascular monitoring to inform interventions and to improve long-term cardiovascular health.
Condensed Abstract.
Before the introduction of highly active antiretroviral therapy (HAART), cardiac mortality and morbidity were common in HIV-infected children. We compared serial echocardiograms from HAART-exposed and HAART unexposed groups of HIV-infected children. In general the HAART-exposed group had more normal measures of left ventricular function and structure compared to the HAART-unexposed group. Long term exposure to HAART in perinatally HIV-infected children was associated with better measurements of LV structure and function than those of children from the pre-HAART era. Cardiac function in HAART-exposed children did decline with increasing follow-up, indicating the need for prospective studies as these children become adults and are increasingly exposed to non-HAART-related cardiovascular risk factors.
PERSPECTIVES.
Competency in Medical Knowledge
Exposure to highly active antiretroviral therapy (HAART) in perinatally HIV-infected children is associated with better echocardiographic measures of cardiac structure and function than those of children from the pre-HAART era.
Competency in Patient Care
Clinicians should emphasize reduction strategies for traditional cardiovascular disease risk factors with perinatally HIV-infected children and their families as well as consider echocardiographic monitoring based on evidence-based studies.
Acknowledgments
We thank the clinical study site investigators and their study staffs for subject recruitment and follow-up data collection: (Kenneth C. Rich, MD, Sulekha Kumar, MD, University of Illinois at Chicago, Chicago, IL; Thomas J. Starc, MD, MPH; Phillip LaRussa, MD, Julie Glickstein, MD, Columbia University, New York, NY; Ricardo H. Pignatelli, MD, Louis I. Bezold MD, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX; Philip LaRussa, MD; Sharon O’Brien, MD, Ellen R. Cooper, MD, Boston Medical Center, Boston, Massachusetts). We would also like to thank the children and families whose participation made this study possible.
Sources of Funding: The CHAART-2 study was supported by grant R01 HL078522 from the National Heart, Lung, and Blood Institute (SEL).
Abbreviations
- ART
antiretroviral therapy
- HIV
human immunodeficiency virus
- LV
left ventricle
- LVFS
LV fractional shortening
- GEE
generalized estimating equation modeling
- HAART
highly active antiretroviral therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors report no relationships relevant to the contents of this paper to disclose.
References
- 1.Fisher SD, Easley KA, Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV multicenter study. Am Heart J. 2005;150(3):439–47. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children: the prospective P2C2 HIV multicenter study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation. 2000;102(13):1542–8. doi: 10.1161/01.cir.102.13.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Easley KA, Orav EJ, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV Multicenter Study. Circulation. 1998;97(13):1246–56. doi: 10.1161/01.cir.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney DL, Perez-Atayde AR, Easley KA, et al. Postmortem cardiomegaly and echocardiographic measurements of left ventricular size and function in children infected with the human immunodeficiency virus. The Prospective P2C2 HIV Multicenter Study. Cardiovasc Pathol. 2003;12(3):140–8. doi: 10.1016/s1054-8807(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiovascular status of infants and children of women infected with HIV-1 (P2C2 HIV): a cohort study. Lancet. 2002;360(9330):368–73. doi: 10.1016/S0140-6736(02)09607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Attar I, Orav EJ, Exil V, Vlach SA, Lipshultz SE. Predictors of cardiac morbidity and related mortality in children with acquired immunodeficiency syndrome. J Am Coll Cardiol. 2003;41(9):1598–1605. doi: 10.1016/s0735-1097(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 7.Luginbuhl LM, Orav EJ, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with HIV infection. JAMA. 1993;269(22):2869–75. [PubMed] [Google Scholar]
- 8.Perez-Atayde AR, Kearney DI, Bricker JT, et al. Cardiac, aortic, and pulmonary arteriopathy in HIV-infected children: the prospective P2C2 HIV multicenter study. Pediatr Dev Pathol. 2004;7(1):61–70. doi: 10.1007/s10024-003-1001-9. [DOI] [PubMed] [Google Scholar]
- 9.Lai WW, Colan SD, Easley KA, et al. Dilation of the aortic root in children infected with human immunodeficiency virus type 1: the prospective P2C2 HIV multicenter study. Am Heart J. 2001;141(4):661–70. doi: 10.1067/mhj.2001.113757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langston C, Cooper ER, Goldfarb J, et al. Human immunodeficiency virus-related mortality in infants and children: data from the Pediatric Pulmonary and Cardiovascular Complication of Vertically Transmitted HIV (P2C2) Study. Pediatrics. 2001;107(2):328–38. doi: 10.1542/peds.107.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Williams PL, Wilkinson JD, et al. Cardiac status of children infected with human immunodeficiency virus who are receiving long-term combination antiretroviral therapy: results from the Adolescent Master Protocol of the Multicenter Pediatric HIV/AIDS Cohort Study. JAMA Pediatr. 2013;167(6):520–7. doi: 10.1001/jamapediatrics.2013.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. N Engl J Med. 1997;336(19):1337–42. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 13.The P2C2 HIV Study Group. The Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted Human Immunodeficiency Virus (P2C2 HIV) Infection Study: design and methods. J Clin Epidemiol. 1996;49(11):1285–94. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavigne JE, Shearer WT, Thompson B, et al. Cardiovascular outcomes of pediatric seroreverters perinatally exposed to HAART: design of a longitudinal clinical study. Cardiovasc Toxicol. 2004;4(2):187–97. doi: 10.1385/ct:4:2:187. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Easley KA, Kaplan S, et al. Reliability of multicenter pediatric echocardiographic measurements of left ventricular structure and function: the prospective P2C2 HIV Study. Circulation. 2001;104(3):310–6. doi: 10.1161/01.cir.104.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 17.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 1985;99(2):445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 18.Brogly SB, Abzug MJ, Watts H, et al. Birth defects among children born to human immunodeficiency virus-infected women: pediatric AIDS clinical trials protocols 219 and 219C. Pediatr Infect Dis J. 2010;29(8):721–7. doi: 10.1097/INF.0b013e3181e74a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher SD, Starc TJ, Guerra V, Williams PL, Wilkinson JD, Lipshultz SE. Declining incidence of systolic left ventricular dysfunction in human immunodeficiency virus-infected individuals treated with highly active antiretroviral therapy. Am J Cardiol. 2016;117(7):1194–5. doi: 10.1016/j.amjcard.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel K, Van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR., 3rd International Maternal Pediatric Adolescent AIDS Clinical Trials 219219C Study Team. The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS. 2012;26(16):2027–37. doi: 10.1097/QAD.0b013e3283578bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel K, Wang J, Jacobson DL, et al. Aggregate risk of cardiovascular disease among adolescents perinatally infected with the Human Immunodeficiency Virus. Circulation. 2014;129:1204–1212. doi: 10.1161/CIRCULATIONAHA.113.001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanco F, San Roman J, Vispo E, et al. Management of metabolic complications and cardiovascular risk in HIV-infected patients. AIDS Rev. 2010;12(4):231–41. [PubMed] [Google Scholar]


