Table 1.
α7 nAChR agonists and PAM in clinical trials.
| Compound | Classification | Potency & efficacy | Animal model on CNS disorders | Indication | Clinical status (Sponsor) |
|---|---|---|---|---|---|
| Tropisetron | Partial agonist | Binding affinity: | Mice: phencyclidine-induced cognitive deficits75. | Pain | Phase IV (completed in 2009) |
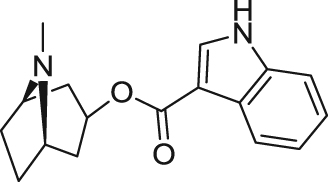 |
Ki: 6.9 nmol/L (in rα7)73 | ||||
| (University Hospital, Clermont-Ferrand) | |||||
| Electrophysiology activity: | Rats: young and aged rats76; naloxone-induced place aversion77. | ||||
| Hα7 in oocytes: EC50 = 0.6 μmol/L; Emax = 25%74 | |||||
| Smoking cessation; schizophrenia | Phase III (completed in 2011) | ||||
| Mα7 in oocytes: EC50 = 1.3 μmol/L; Emax = 36%73 | |||||
| (Baylor College of Medicine) | |||||
| GTS-21/DMXB-A | Partial agonist | Binding affinity: | Rats: normal or isoflurane-induced cognitive impairment aged rats80., 81., 82.; ibotenic acid-induced dementia83; mecamylamine-caused learning impairment84; auditory gating in isolation-reared rats85; apomorphine/MK-801-elicited PPI deficits86., 87.. | Schizophrenia | Phase II (completed in 2015) |
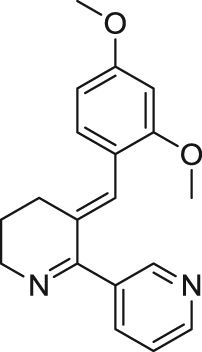 | |||||
| Ki: 2000 nmol/L (in hα7)78 | |||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 11.0 μmol/L; Emax = 9%79 | (University of Colorado) | ||||
| Rα7 in oocytes: EC50 = 5.2 μmol/L; Emax = 32%79 | AD; ADHD | Phase II (completed in 2008) | |||
| (CoMentis) | |||||
| Mice: Aβ-induced cognitive deficits45; deficient sensory inhibition88; aggressive behavior in mouse models89. | |||||
| Tobacco use disorder | Phase II (not yet recruiting) | ||||
| Rabits: aged rabbits90., 91.. | |||||
| (University of Florida) | |||||
| Monkeys: normal monkeys in DMTS task78; Ketamine-induced cognitive deficit92. | |||||
| ABT-126 | Agonist | Binding affinity: | Monkeys: Parkinsonian monkeys95. | AD | Phase II (terminated in 2014) |
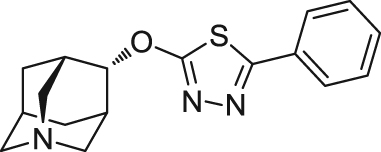 |
Ki: 12–14 nmol/L (in hα7, rα7 and mα7)93., 94. | ||||
| (AbbVie) | |||||
| Schizophrenia | Phase II (terminated in 2014) | ||||
| (AbbVie) | |||||
| AZD0328 | Partial agonist | Binding affinity: | Mice: NOR in normal mice96., 97.. | AD | Phase I (completed in 2008) |
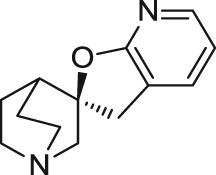 |
Ki: 3.0 and 4.7 nmol/L (in hα7 and rα7)96 | ||||
| Electrophysiology activity: | Monkeys: normal monkeys in delayed response task98. | ||||
| Hα7 in oocytes: EC50 = 338 nmol/L; Emax = 64.7%96 | (AstraZeneca) | ||||
| Schizophrenia | Phase II (terminated in 2008) | ||||
| Rα7 in oocytes: EC50 = 150 nmol/L; Emax = 61.0%96 | |||||
| (AstraZeneca) | |||||
| BMS-933043 | Partial agonist | Binding affinity: | Rats: MK-801-induced cognitive deficits73; S(+)ketamine-induced sensory gating deficits73. | Schizophrenia | Phase I (completed in 2013) |
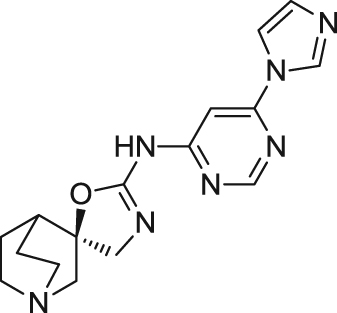 |
Ki: 8.1 and 3.3 nmol/L (in hα7 and rα7)73 | ||||
| Ca2+ flux assays: | |||||
| Hα7 in HEK293 cell line: EC50 = 23.4 nmol/L73 | (Bristol-Myers Squibb) | ||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 0.29 μmol/L;Emax = 24%73 | |||||
| Rα7 in oocytes: EC50 = 0.14 μmol/L; Emax = 27%73 | |||||
| Mice: MK-801-induced cognitive deficits73. | |||||
| EVP-6124/ Encenicline | Partial agonist | Binding affinity: | Rats: scopolamine-induced deficit99; delay-dependent forgetting in the NOR100; low attentive rats101. | AD; dementia | Phase III (terminated in 2017) |
| Ki: 9.98 nmol/L (in rα7)99 | |||||
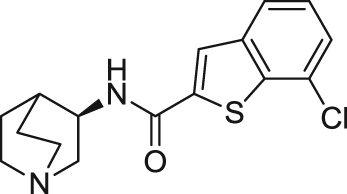 |
(FORUM) | ||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 0.39 μmol/L; Emax = 42%99 | Schizophrenia; impaired cognition | Phase III (completed in 2016) | |||
| (FORUM) | |||||
| Nicotine dependence; smoking cessation | Phase II (terminated in 2015) | ||||
| (A. Eden Evins) | |||||
| MEM3454/RG3487 | Partial agonist | Binding affinity: | Rats: attentional performance in normal rats103; aged rats102; apomorphine-induced deficits in sensorimotor gating102. | AD | Phase II (completed in 2007) |
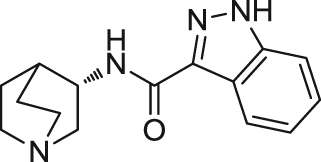 |
Ki: 6 nmol/L (in rα7)102 | ||||
| Electrophysiology activity: | (Memory) | ||||
| Hα7 in oocytes: EC50 = 0.8 μmol/L; Emax = 63%102 | |||||
| Schizophrenia | Phase II (unknown) | ||||
| Hα7 in QM cell line: EC50 = 7.7 μmol/L; Emax = 69%102 | (Memory) | ||||
| AQW051 | Partial agonist | Binding affinity: | Rats: aged rats05105 | Schizophrenia | Phase II (completed in 2013) |
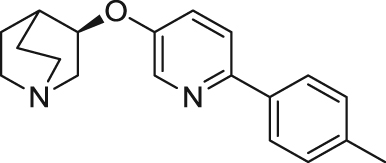 |
Ki: 27 nmol/L104 | ||||
| Ca2+ flux assays: | Mice: NOR in normal mice105 | ||||
| Hα7: EC50 = 7.4 μmol/L; Emax = 73%105 | Monkeys: Parkinsonian monkeys106 | (Novartis) | |||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 7.5 μmol/L; Emax = 75%105 | Levodopa-induced dyskinesia in PD | Phase II (completed in 2013) | |||
| (Novartis) | |||||
| AD | Phase II (terminated in 2009) | ||||
| (Novartis) | |||||
| TC-5619 | Full agonist | Binding affinity: | Mice: th(tk–)/th(tk–) mice108; apomorphine-induced PPI deficits108; NOR in normal mice108. | Schizophrenia | Phase II (completed in 2013) |
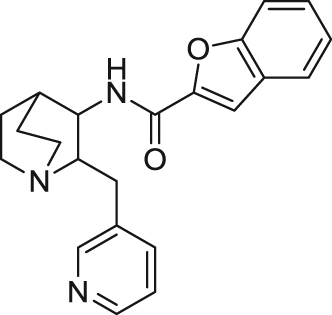 | |||||
| Ki: 1 and 1.4 nmol/L (in hα7 and rα7)107., 108. | |||||
| Electrophysiology activity: | (Targacept) | ||||
| Hα7 in oocytes: EC50 = 33 nmol/L; Emax = 100%108., 109. | AD | Phase I (completed in 2011) | |||
| Rα7 in GH4C1 cell line: EC50 = 17 nmol/L; Emax = 76%107 | (Targacept) | ||||
| ADHD | Phase II (completed in 2012) | ||||
| (Targacept) | |||||
| SSR-180711 | Partial agonist | Binding affinity: | Rats: MK-801/PCP-induced cognitive deficits111; depressive disorders rates111; neurodevelopmental latent inhibition models of schizophrenia112. | AD | Phase II (terminated in 2008) |
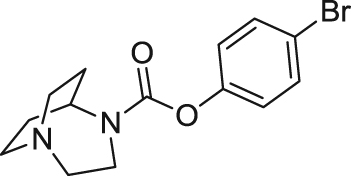 |
Ki: 14 and 22 nmol/L (in hα7 and rα7)110 | ||||
| (Sanofi) | |||||
| Electrophysiology activity: | |||||
| Hα7 in oocytes: EC50 = 4.4 μmol/L; Emax = 51%110 | Mice: chronic mild stress model113; Aβ-induced memory deficits114; phencyclidine-induced cognitive deficits115; forced swim and tail suspension tests116 | ||||
| Hα7 in GH4C1 cell line: EC50 = 0.9 μmol/L; Emax = 36%110 | |||||
| APN1125 | Partial agonist | Electrophysiology activity: | Rats: NOR in normal rats117 | Schizophrenia | Phase I / Phase II (suspended in 2016) |
| (Structure Undisclosed) | Hα7 in oocytes: EC50 = 1.16 μmol/L; Emax = 41%117 | ||||
| (CoMentis) | |||||
| AVL-3288/XY4083/CCMI | Type I PAM | Electrophysiology activity: | Mice: DBA/2 mouse model of sensory-gating deficit118; MK-801-induced hyperlocomotion mode eight-arm radial maze in normal mice118; NOR in normal mice119. | Schizophrenia; schizoaffective disorder | Phase I (recruiting) |
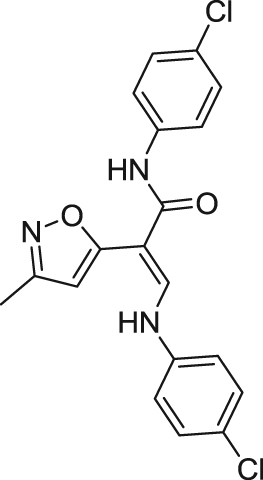 | |||||
| Hα7 in oocytes: EC50 = 0.7 μmol/L; Emax = 9 folds118 | (New York State Psychiatric Institute; University of Colorado) | ||||
| Rats: 5-CSRTT in normal rats119; ketamine-induced cognitive deficits and social withdrawal120. |
DMTS, delayed matching-to-sample; NOR, novel object recognition; PCP, neonatal phencyclidine; 5-CSRTT, five-choice serial reaction time task.
Indications and clinical status of α7 nAChR modulators above are obtained from https://clinicaltrials.gov/.
