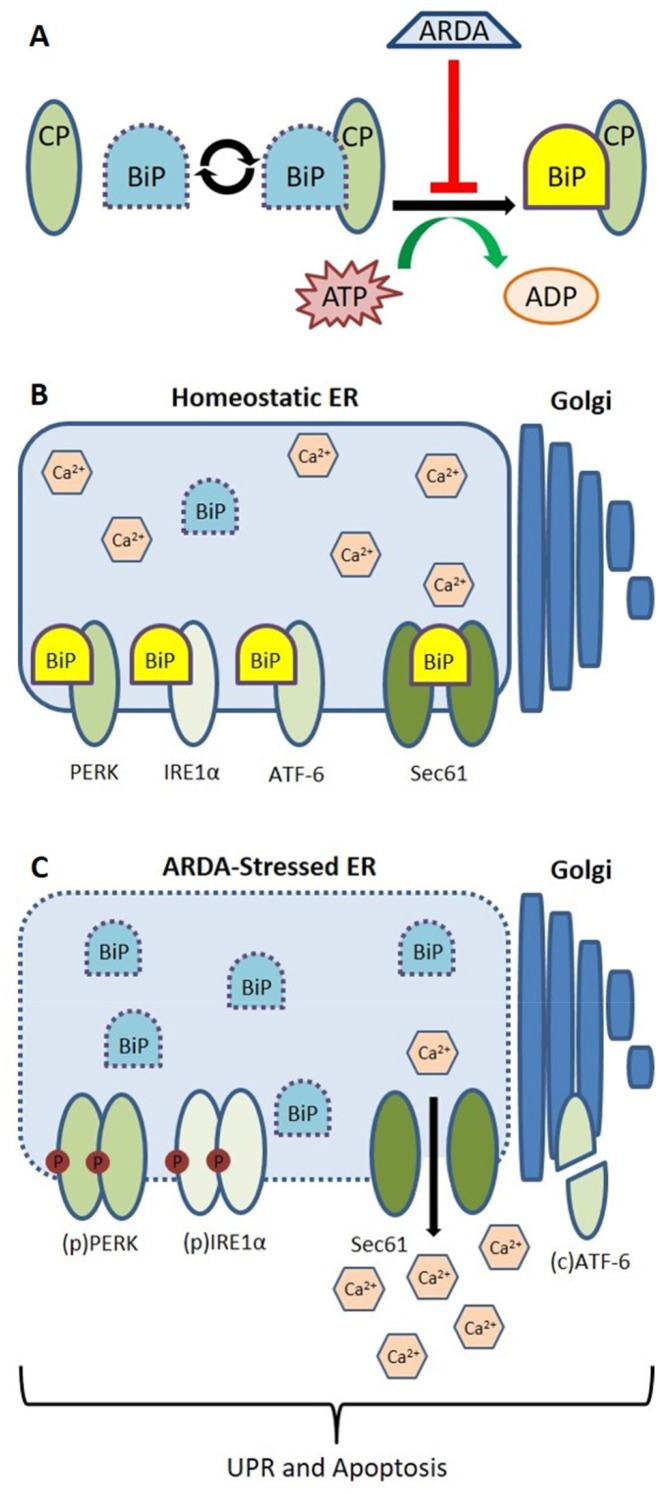
Figure 7. Illustrative model predicting ARDA-mediated disruption of BiP/Grp78 function and consequent induction of the UPR.
(A) When bound to ATP, BiP/Grp78 interacts transiently with its client proteins (CP). Hydrolysis of ATP to ADP drives the formation of stable (yellow) BiP•CP complexes by altering the conformation and affinity of BiP’s SBD. The ARDAs VNPT-178 and VNLG-74A bind to BiP’s ATPase domain and prevent association of stable complexes. (B) BiP maintains ER homeostasis through its stable interactions with ER transmembrane proteins PERK, IRE1α, ATF-6 and the Sec61 translocon. (C) Administration of ARDA causes a UPR and ultimately apoptosis by promoting the dissociation of BiP/Grp78 from its client proteins resulting in calcium efflux through the Sec61 translocon, the oligomerization and phosphorylation of PERK and IRE1α as well as ATF-6’s translocation to and cleavage within the Golgi.