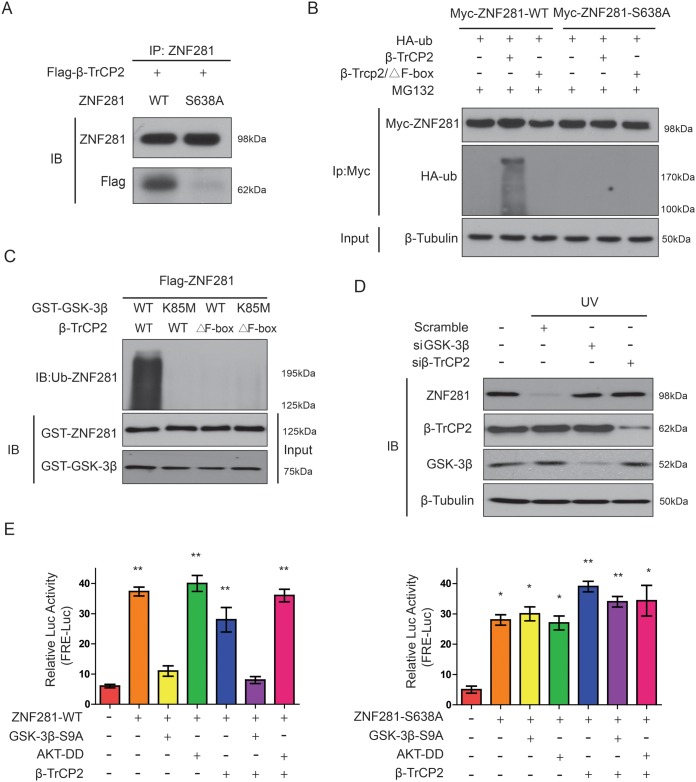
Figure 7. Phosphorylation of ZNF281 at Ser638 by GSK-3β promotes its binding to β-TrCP2 and degradation.
(A) Flag-β-TrCP2 was cotransfected with WT-ZNF281 or mutant ZNF281-S638A into HCT116 cells then treated with MG132 (10 μM) for 10 h. Flag-β-TrCP2 was immunoprecipitated and then analyzed for ZNF281. (B) HCT116 cells were transfected with indicated plasmids, and then treated with MG132 (10 μM). ZNF281 was immunoprecipitated and then analyzed with anti-HA-ubiquitin (HA-ub). (C) Purified ZNF281 protein was phosphorylated by GSK-3β and then incubated with in vitro-translated F-box-deleted β-TrCP2 and β-TrCP2 or in the presence of other indicated plasmids. ZNF281 ubiquitination was analyzed. (D) SiRNA against GSK-3β or β-TrCP2 were transfected for 48 h, and then HCT116 cells were stimulated with UV irradiation. ZNF281 phosphorylation was determined by Western blotting. (E) 293T cells cotransfected with firefly luciferase reporter containing ZNF281-responsive elements, pRL-TK as a transfection control for normalization), and other indicated plasmids, then samples were analyzed. *P <0.05, **P <0.01.