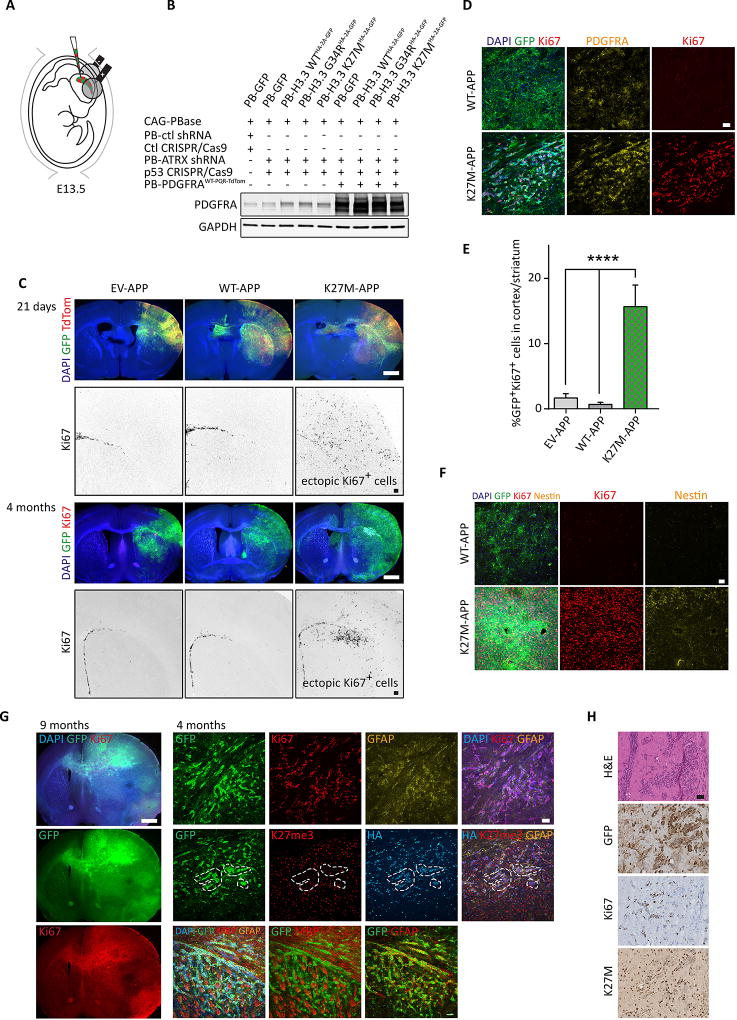
Figure 3. Addition of PDGFRAWT overexpression to H3.3K27M and ATRX/p53 KD results in shorter latency of tumorigenesis.
(A) Schematic describing the in utero electroporation strategy used to deliver piggyBac transposable-empty vector, H3.3WT or H3.3K27M along with PDGFRAWT, ATRX shRNA and Trp53 CRISPR/Cas9 (EV/WT/K27M-APP) into cortical NPCs in vivo. (B) Validation of PDGFRAWT overexpression in ex vivo NPCs sorted and expanded 3 days following electroporation. (C) Coronal sections of EV-APP, WT-APP and K27M-APP brains prepared 21 days and 4 months following electroporation showing immunofluorescent detection of GFP, TdTomato, Ki67 and DAPI. Scale bars represent 1.5 mm (multi-color panels) and 200 µm (black-and-white panels). (D) Immunofluorescent overlap between GFP+, Ki67+ and PDGFRA in WT-APP and K27M-APP brains. Scale bars represent 50 µm. (E) Quantification of GFP+Ki67+ cells in EV-APP, WT-APP and K27M-APP electroporated brains at 4 months. Data are represented as mean ± SEM. (F) Comparison of Ki67 and Nestin immunofluorescence levels in WT-APP and K27M-APP at 9 months following surgery. Scale bars represent 50 µm. (G) Left: low magnification view of K27M-APP tumors at 9 months. Right: immunofluorescence analysis of K27M-APP tumors, as depicted, 4 months following surgery. Scale bars represent 1.5 mm (left) and 50 µm (right). (H) Histology of K27M-APP tumors. Scale bars represent 100 µm. *p<0.05, **p<0.01, ****p<0.0001. See also Figure S3 and Table S1.