Figure 5. Structural and functional homology of Strigolactone and Karrikin receptors.
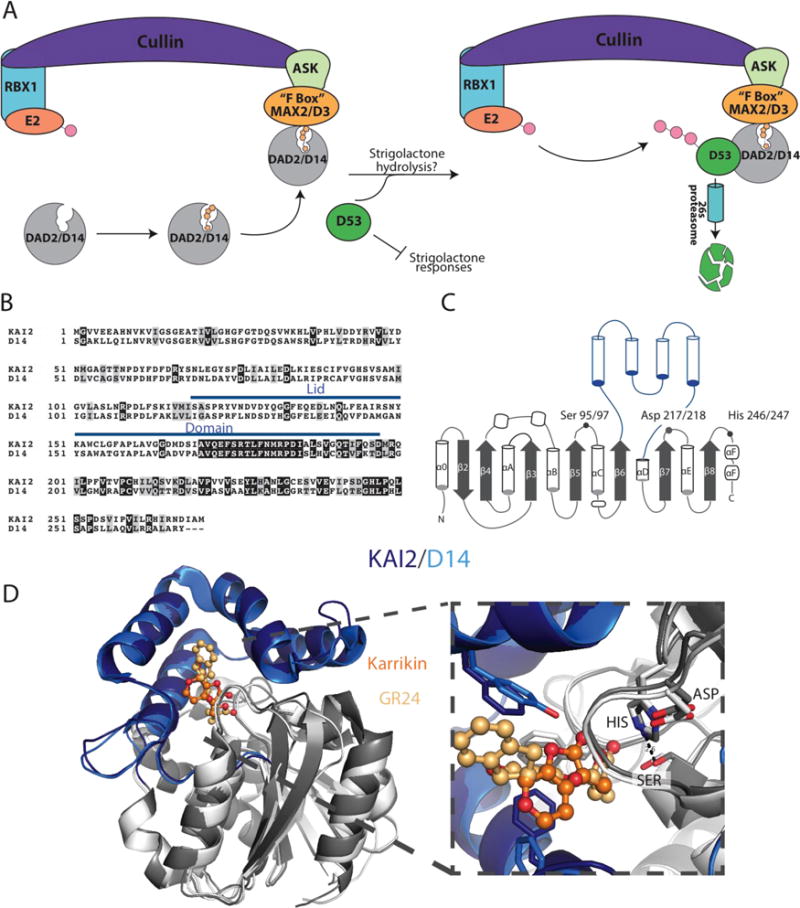
A) Proposed signaling pathway for D14/DAD2. D14/DAD2 binds strigolactone and is recruited to the SCF E3 ligase associated FBOX protein, MAX2, in order to form a complex between DAD2/D14, MAX2, and D53. SCF(SKP1-like Cul1 F-box) E3 ubiquitin-ligase complex are comprised of Cullin, the RING-H2 protein RBX1, a ubiquitin conjugating enzyme E2, and an SKP1-like protein that acts as a substrate adaptor to recruit specific FBOX proteins. FBOX proteins are named for their 50 amino acid FBOX domain, originally identified in the mammalian cyclin F protein, and are responsible for recruiting E3 ligase substrates that undergo 26s proteasomal degradation through polyubiquitinylation.[61] Upon recruitment by MAX2, D53 is then polyubiquitinylated and destroyed through the 26s proteasome pathway, thereby removing inhibition of strigolactone responses. The underlying importance of strigolactone hydrolysis and signal transduction is still not well understood and is therefore left open to interpretation. The above figure does not take into account that D14/DAD2 can independently bind with D53 or MAX2/D3 in the presence of strigolactone, which may indicate that D14/DAD2 interacts with D53 before MAX2/D3. B) Sequence alignment of A. thaliana KAI2(PDB: 4jym) and O. sativa D14 (PDB: 5dj5), with the lid domains indicated with a blue line. Identical and similar positions are indicated in black and grey boxes, respectively. C) Structural topology of KAI2 and D14 with the lid domains indicated in blue helices and the core domain in grey. The catalytic triad residues are labeled; the positions are numbered in the order, Kai2/D14. D) X-ray crystal structures of Kai2 and D14 are shown as cartoon diagrams and superimposed. Lid domains are colored in dark blue (KAI2) and light blue (D14), core domains in dark grey (KAI2) and light grey (D14). Zoom in picture of the active site shows the catalytic triads and ligands Karrikin (bright orange) and the strigolactone analog, G24 (light orange).
