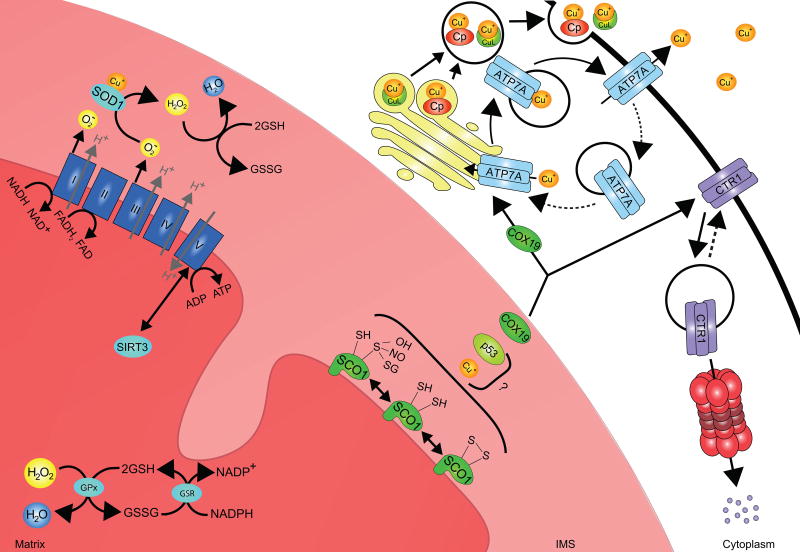
Figure 2. Potential inputs that contribute to SCO1-dependent mitochondrial signaling and the regulation of copper homeostasis.
SCO1 function is integral to a mitochondrial signaling pathway that impinges upon copper homeostasis. The redox state of its cysteinyl sulphurs, which are located within the Cx3C motif, is significantly perturbed in patients with combined COX and total copper deficiencies. Thus, signaling may be directly modulated by the proportion of the SCO1 pool with copper-loaded versus apo-Cx3C motifs, or by the balance of reversible post-translational modifications to one or both of its cysteinyl sulphurs (S-S, SH, SOH, SNO or SSG). How the functional status of SCO1 is sensed and transduced outside of the mitochondrion to effect changes in the abundance or localization of the copper import or efflux machinery remains poorly understood. COX19 partitions between the IMS and the cytosol in a copper-dependent manner, and acts alone or in concert with unknown factors to stimulate ATP7A–mediated copper export from the cell by regulating its trafficking to the plasma membrane and/or increasing the rate of secretion of copper bound to cupro-proteins (e.g Cp, ceruloplasmin) or small molecules (e.g. CuL, copper ligand). Mechanisms that couple SCO1-dependent mitochondrial signaling to the regulation of CTR1 localization or degradation are currently unknown. However, redox and/or metabolic switch mechanisms may contribute to the modulation of SCO1-dependent mitochondrial signaling. Complexes (labeled I-IV) of the electron transport chain (ETC) oxidize NADH and FADH2 while establishing a proton gradient that is harnessed by Complex V to generate ATP. Perturbations in this pathway lead to changes in the NAD+:NADH and AMP:ATP ratios and the activation of the AMPK/p53 pathway and SIRT3, both of which trigger downstream changes in metabolism that may affect cellular copper levels. Complexes I and III of the ETC also generate superoxide anions and ROS are known to promote signaling by post-translationally modifying proteins and/or altering redox balance. Changes in the mitochondrial redox state are sensed primarily by the GSH:GSSG ratio, which is buffered within the matrix by the activity of the enzymes glutathione peroxidase (GPx) and glutathione reductase (GSR).