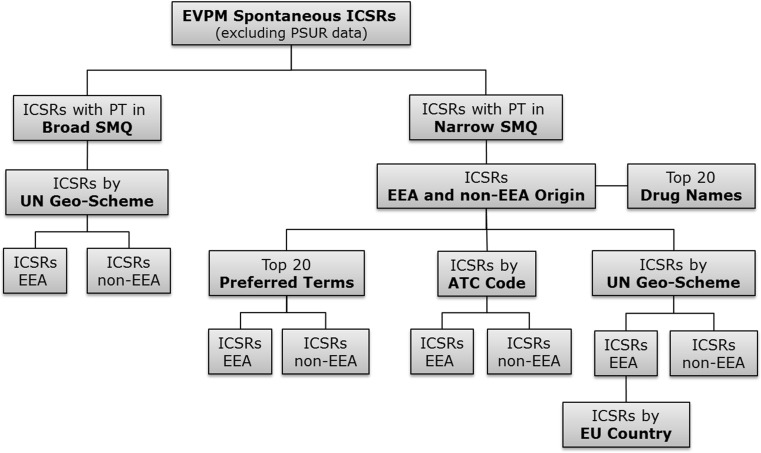
Fig. 1.
EudraVigilance ICSR data extraction pathway using the broad and narrow SMQ for medication errors. Non-serious PSUR cases were excluded. ICSR individual case safety reports, SMQ standardised MedDRA® Query, EVPM eudravigilance post-authorisation module, PT preferred terms, EEA European economic area, ATC anatomical therapeutic classification, PSUR periodic safety update report, UN United Nations, MedDRA ® medical dictionary for regulatory activities