Figure 2. Characterizing mvsFlt conjugation efficiency and size.
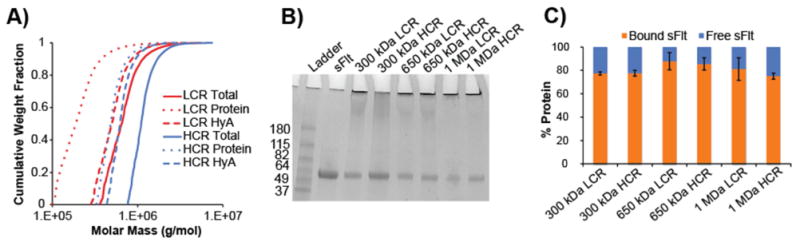
A) SEC-MALS chromatogram depicting cumulative weight fraction versus molar mass of 650 kDa LCR and HCR bioconjugates. Dotted line, dashed line, and solid line represent total molar mass of all covalently attached sFlt proteins, HyA molar mass and total bioconjugate molar mass (all given as g/mol), respectively. B) 4-20% SDS-page gradient gel of sFlt and mvsFlt. Protein bands in the stacking gel indicate successful protein conjugation to HyA. Protein bands within gel represent the proportion of protein that was not covalently bound, but remained in solution after dialysis. C) Quantified protein band intensities of SDS-PAGE gel. Percent bound sFlt was determined by dividing the intensity of protein in the stacking gel by total protein intensity within the respective well. Free sFlt was determined by dividing the intensity of protein within the separating gel by the total protein intensity within the respective well.
