Abstract
Background
Bone tissue engineering, a powerful tool to treat bone defects, is highly dependent on use of scaffolds. Both silk fibroin (SF) and chitosan (Cs) are biocompatible and actively studied for reconstruction of tissue engineering. Gelatin (Gel) is also widely applied in the biomedical field due to its low antigenicity and physicochemical stability.
Material/Methods
In this study, 4 different types of scaffolds were constructed – SF, SF/Cs, SF/Gel, and SF/Cs/Gel – and we compared their physical and chemical properties as well as biological characterization of these scaffolds to determine the most suitable scaffold for use in bone regeneration. First, these scaffolds were produced via chemical cross-linking method and freeze-drying technique. Next, the characterization of internal structure was studied using scanning electron microscopy and the porosity was evaluated by liquid displacement method. Then, we compared physicochemical properties such as water absorption rate and degradation property. Finally, MC3T3-E1 cells were inoculated on the scaffolds to study the biocompatibility and osteogenesis of the three-dimensional (3D) scaffolds in vitro.
Results
The composite scaffold formed by all 3 components was the best for use in bone regeneration.
Conclusions
We conclude that the best scaffold among the 4 studied for MC3T3-E1 cells is our SF/Cs/Gel scaffold, suggesting a new choice for bone regeneration that can be used to treat bone defects or fractures in clinical practice.
MeSH Keywords: Chitosan, Fibroins, Gelatin, Tissue Engineering, Tissue Scaffolds
Background
Bone defects or fractures occur in many ways, including trauma, neoplasm, congenital defects, motor vehicle accidents, osteoporosis, and arthritis [1]. The rising incidence of bone disorders has resulted in the need for more effective therapies to meet this demand, exacerbated by an increasingly aging population [2]. Bone tissue engineering has become a significant alternative to conventional bone grafts for bone reconstruction, and the production of a scaffold with high cell affinity and osseointegrative properties is crucial for successful bone substitutes [3].
There are a variety of methods, including gas foaming [4], particulate-leaching [5], electrospinning [6], and freeze-drying [7], to fabricate scaffolds that meet several requirements considered essential for bone regeneration. The materials must have good biocompatibility and cell affinity, as well as the ability to sustain osteoblast extracellular matrix.
Silk fibroin (SF), chitosan (Cs), and gelatin (Gel) are good scaffold materials for bone tissue engineering. SF is a fibrous protein which is produced mainly by silkworms and spiders and has unique mechanical properties, tunable biodegradation rate, and the ability to support the differentiation of mesenchymal stem cells along the osteogenic lineage [8]. Cs is a linear polysaccharide derived from partial de-acetylation of chitin and is considered an effective biomaterial for tissue engineering because it has many excellent properties such as antibacterial activity, nontoxicity, and hemostatic effect [9,10]. Gelatin is a soluble protein derived from partially denatured collagen, with attractive properties such as good biocompatibility, low immunogenicity, plasticity, adhesiveness, promotion of cell adhesion and growth, and low cost, making it ideally suited as a biomaterial for tissue engineering [11]. There have been several articles concerning these materials [12–14], Kim et al. studied silk fibroin/titanium dioxide/hydroxyapatite hybrid scaffold, Maji et al. studied gelatin-chitosan-hydroxyapatite scaffold, and Rajzer et al. developed a scaffold composed of gelatin and poly (ɛ-caprolactone) fibrous and calcium phosphate. However, few studies have assessed the SF/Cs/Gel scaffold, although Gel has great biocompatibility, and the optimal scaffold for bone tissue regeneration is still unknown.
In this study, we produced 4 different types of scaffolds (SF, SF/Cs, SF/Gel, and SF/Cs/Gel) and compared them to determine which is best for use in bone reconstruction.
Material and Methods
Materials
Cocoons of Bombyx mori (silkworm) were provided by the College of Textile and Clothing Engineering, Soochow University. Chitosan powders with molecular weight about 900 000 Da and 95% degree of deacetylation were purchased from Jiangsu Aoxin Biotechnology Co., Ltd. Gelatin, sodium carbonate, lithium bromide, acetic acid, aqueous ethanol, polyethylene glycol 6000, dimethylsulfoxide (DMSO), MTT, 4% paraformaldehyde, hematoxylin, eosin, crystal violet, and dialysis bags were obtained from Shanghai Yuanye Biotechnology Co., Ltd. Ethylene dichloride (EDC) and N-Hydroxysuccinimide (NHS) were acquired from Adamas Reagent, Ltd. MEM, FBS, and penicillin–streptomycin were bought from Thermo Fisher Scientific Inc.
Scaffold production
Preparation of silk fibroin solution
Silk fibroin was derived from cocoons of Bombyx mori through degumming. First, cocoons were finely chopped into pieces and then boiled in 5% sodium carbonate at 100°C for 1 h. Next, the silk fibroin was washed with distilled water and fully dried. This procedure was repeated 3 times to ensure that silk sericin was removed completely.
Silk fibroin solution was prepared in the following steps. First, degummed silk fibers were dissolved with 9 M lithium bromide at 80°C and subjected to magnetic stirring until they were completely dissolved. After it was cooled to room temperature, the solution was filtered to remove insolubles. Then, the solution was dialyzed against distilled water in a dialysis bag with a molecular weight cut-off (MWCO) of 3500 Da at 4°C for 3 days. The water was changed every 3 h to remove small molecules from the silk fibroin solution.
The final step was to control the concentration of silk fibroin solution. We placed the dialysis bag containing the silk fibroin solution into polyethylene glycol 6000 powders, dried and concentrated to collect the liquid. Then, 3 beakers were washed clean and carefully dried to measure the concentration of the solution. The 3 beakers were fully cooled and weighed (M1). In each beaker, 5 ml silk fibroin solution was added and weighed again (M2). The beakers were then placed into a 60°C oven until the solution was dried and weighed after cooling (M3). The following formula was used to determine the concentration:
The mean concentration was calculated and the concentration of the final silk fibroin solution was adjusted to be 3%.
Preparation of chitosan solution
To prepare 3% chitosan solution, 3 g chitosan powders were dissolved in 100 ml 1% acetic acid solution and subjected to magnetic stirring until they were completely dissolved. The solution was then filtered to remove impurities.
Preparation of gelatin solution
For preparation of 3% gelatin solution, 3 g gelatin particles were dissolved in 100 ml 1% acetic acid solution in a 50°C water bath and stirred until they were completely dissolved.
Production of composite scaffold
Different types of 3D scaffolds were produced via freeze-drying technique and chemical cross-linking method. SF scaffolds were produced with silk fibroin solution only; SF/CS scaffolds were manufactured using a 1: 1 ratio (w/w) of silk fibroin solution and chitosan solution; SF/Gel scaffolds were produced with a ratio of 1: 1 (w/w) of silk fibroin solution and gelatin solution; and a 2: 1: 1 ratio (w/w) of silk fibroin, chitosan, and gelatin was used for SF/CS/Gel scaffolds.
All the mixtures were then added to 95% aqueous ethanol solution containing 50 mmol/L ethylene dichloride (EDC) and 18 mmol/L N-Hydroxysuccinimide (NHS) and maintained under magnetic stirring for 15 min. Next, 1 ml of the final solution was transferred to a well of a 24-well plate and was subjected to cross-linking at 4°C for 24 h.
After chemical cross-linking, the 24-well plates were frozen at −20°C for 24 h before being placed into a −80°C freezer for 24 h, and they were maintained in a freeze-drying machine for 48 h to produce the scaffolds. Before cell culture, the scaffolds were sterilized with radiation.
Characterization of the structure
Macroscopic appearance
After being produced, different types of 3D scaffolds were removed from the wells and placed together for comparison and photography.
Internal morphology
The internal structures and morphology of the scaffolds were measured with a scanning electron microscope after gold spraying.
Porosity evaluation
The porosity of the scaffolds was examined by the liquid displacement method [15,16]. A scaffold was immersed in water of known volume (V1) for 10 min. The total volume of water and the water-impregnated scaffold was recorded as V2. The scaffold was then removed, and the residual liquid volume was recorded as V3. The porosity of this scaffold was obtained by the following equation: Porosity (%)=(V1–V3)/(V2–V3)×100%. The experiments were performed 3 times, and the average porosity value was obtained.
Physical and chemical properties
Water absorption rate
The dry scaffold was weighed (W1) and then placed in distilled water until fully impregnated. The surface water was removed with filter paper and weighed (W2). The water absorption rate was calculated by the following equation: Water absorption rate (%)=(W2–W1)/W1×100%. The experiments were performed 3 times, and the average water absorption rate was obtained.
Degradation property
Scaffold blocks were weighed (W0) and then placed in the culture medium in 15-ml centrifuge tubes. Next, the centrifuge tubes were placed in a moisturizing thermostat at 37°C. After that, the samples were dried at 60°C and weighed at 1 d (day), 3 d, 7 d, and 10 d (Wn). Each weight was determined 3 times, and the average weight was substituted into the following equation in order to calculate the degradation ratio of each scaffold at different time points: Degradation ratio (%)=(W0–Wn)/W0×100%.
Biological characterization
MC3T3-E1 cells are preosteoblasts from mice. We used MC3T3-E1 to study biocompatibility and osteogenesis of the scaffolds in vitro to confirm the optimal one for use in bone tissue engineering.
Cell culture
The culture medium of MC3T3-E1 consisted of MEM supplemented with 10% FBS and 1% penicillin–streptomycin. Prior to cell seeding, the scaffolds were sterilized with radiation for 48 h and then pre-wetted in culture medium for 24 h. Then, 1 ml of cell suspension (5×104/ml cells except in the adhesion rate test, where 1×105/ml cells were used) was dropped onto the scaffold material and then agitated for 5 min so that the cells were seeded uniformly in the pores of the 3D scaffold. The 4 groups were then placed in the same incubator for continuous culture.
Cell adhesion rate
A 1-ml cell suspension containing 105 cells (A0) were seeded on the pre-wetted scaffolds and the adhesion rates were measured at 1, 3, and 6 h after inoculation. The scaffold was removed from the well and the cell number was counted with a cell-counting board (A1). After removing all the medium solution, cells adhered to the well wall were digested and calculated (A2). The cell adhesion rate was confirmed with the following equation: Adhesion rate (%)=(A0–A1–A2)/A0×100%. The experiments were performed 3 times per group, and the average adhesion rate was obtained.
Cell proliferation rate
We measured cell the proliferation rate of each group with a method called MTT. A 1-ml cell suspension containing 5×104 cells was inoculated on the pre-wetted scaffold. At 1, 3, 5, and 7 days after inoculation, we examined cell proliferation rate using the following steps. First, to each well we added 1 ml dimethylsulfoxide (DMSO) and then placed it in the incubator for 4 h. Next, 200 μl MTT was added in the dark and the plate was placed in the incubator for 30 min. After that, the plate was agitated for 15 min before we removed the scaffolds and shifted 120 μl mixture per well to a 96-well-plate. The 96-well plate was then placed in a microplate reader to measure the absorbance (OD value) at 560 nm. The control group was a 2D cell culture system.
Cell growth and morphology
One ml of cell suspension containing 5×104 cells was inoculated on the pre-wetted scaffolds and the morphology of the cells was detected at 1, 3, 5, and 7 days after inoculation through the following methods.
Scanning electron microscopy
A scanning electron microscope (SEM) is a type of electron microscope that can produce internal images of a sample to obtain morphology of cells on the scaffolds. The culture medium was sucked out and the scaffolds were rinsed with PBS 3 times before 1 ml of 4% paraformaldehyde solution was added for fixation. Then, 4 h later, the plates were frozen at −20°C for 24 h before being placed into a −80°C freezer for 24 h, and finally they were put into a freeze-drying machine for 48 h to freeze-dry the scaffolds. Cell morphology was examined with a scanning electron microscope.
Staining
We sorted the optimal scaffold which had been detected to culture the most cells in the same conditions to further our research by carrying out hematoxylin staining, hematoxylin-eosin staining, and crystal violet staining to study cell morphology. The scaffolds were rinsed with PBS and fixed with 1 ml of 4% paraformaldehyde solution before hematoxylin staining, hematoxylin-eosin staining, and crystal violet staining. After staining, we observed the images through a phase-contrast microscope.
Results
Characterization of the structure
Macroscopic appearance
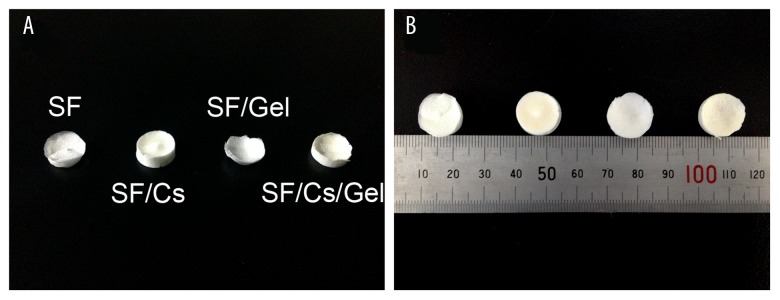
The macroscopic appearance of SF, SF/Cs, SF/Gel, and SF/Cs/Gel scaffolds is shown in Figure 1. Without using microscopy, we found SF and SF/Gel scaffolds to be pure white, while SF/Cs and SF/Cs/Gel scaffolds were yellowish-white, and scaffolds containing gelatin were more smoother and harder.
Figure 1.
Macroscopic appearance of SF, SF/Cs, SF/Gel, and SF/Cs/Gel scaffolds observed from above (A) and from top to bottom (B).
Scanning electron microscopy
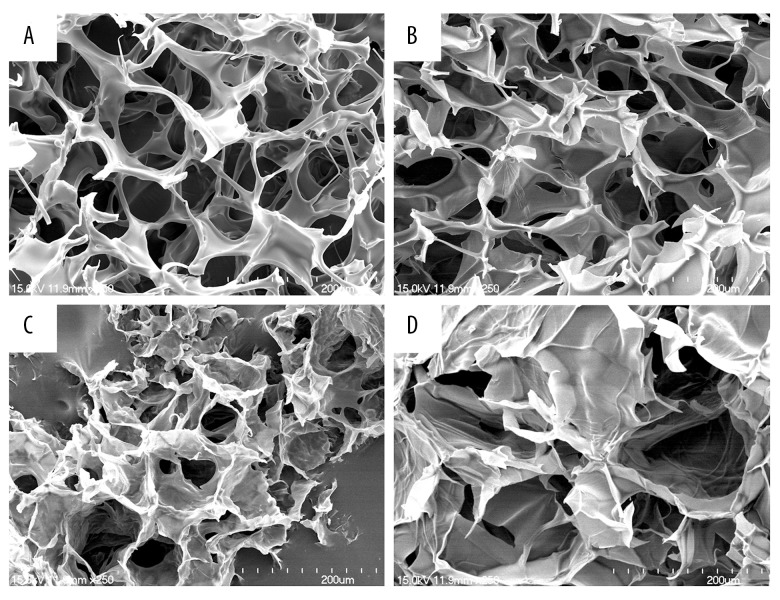
We used scanning electron microscope to study the internal structure of the scaffolds. The SEM images of SF, SF/Cs, SF/Gel, and SF/Cs/Gel scaffolds are shown in Figure 2. All 4 scaffolds had porous network structures and good connectivity between the pores. However, they had different pore sizes and internal structures. The SF scaffold and SF/CS scaffold had uniform pore sizes ranging from 40 to 60 μm, while the pores of the SF/Gel scaffold ranged from 20 to 100 μm. The SF/CS/Gel scaffold had larger pore size, ranging from 100 to nearly 200 μm.
Figure 2.
SEM images of SF, SF/Cs, SF/Gel and SF/Cs/Gel scaffolds. All the scaffolds were magnified 250 times and the scale bars are 200 μm. (A) SF. (B) SF/Cs. (C) SF/Gel. (D) SF/Cs/Gel.
Porosity evaluation
The porosities of the scaffolds were all greater than 40% (Table 1). Of all the scaffolds, the SF/Cs scaffold had the highest porosity, at 77.48±1.27%, while the SF scaffold ranked second with a porosity of 60.74±3.23%. The porosities of the SF/CS/Gel scaffold and SF/Gel scaffold were 42.06±4.83% and 41.67±8.33%, respectively.
Table 1.
Porosities and water absorption rates of scaffolds presented as means ± standard deviation (n=3).
| Scaffold | Porosity (%) | Water absorption rate (%) |
|---|---|---|
| SF | 60.74±3.23 | 1419.99±8.19 |
| SF/Cs | 77.48±1.27 | 3855.75±17.40 |
| SF/Gel | 41.67±8.33 | 372.94±1.41 |
| SF/Cs/Gel | 42.06±4.83 | 537.34±1.61 |
Physical and chemical properties
The physical and chemical properties of the scaffolds that were estimated in this study contained water absorption rate and degradation property.
Water absorption rate
The water absorption rates of the scaffolds are illustrated in Table 1. Water uptake rates of the scaffolds in the distilled water were above 350%, regardless of the scaffold type. The SF/Cs scaffold had the highest water uptake rate, at 3855.75±17.40%.
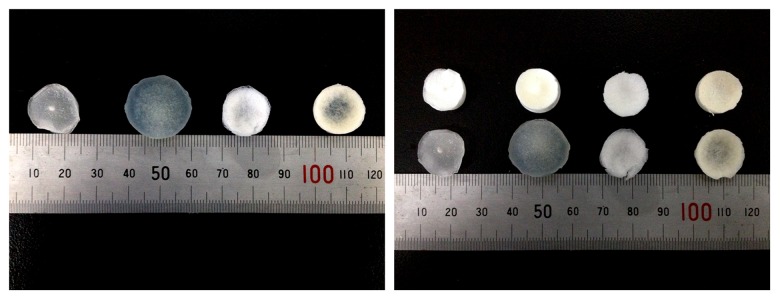
The macroscopic appearance of the wet scaffolds is shown in Figure 3. It is obvious that all the 4 scaffolds had larger volumes after water uptake, and the SF/Cs scaffold was much larger than before, which shows its high water absorption rate.
Figure 3.
The macroscopic appearance of the wet scaffolds.
Degradation property
The degradation rate of the scaffolds immersed in culture medium is shown in Figure 4. The SF scaffold had the highest degradation rate and the SF/Cs/Gel scaffold ranked second. The SF/Cs scaffold and SF/Gel scaffold had similar degradation properties.
Figure 4.
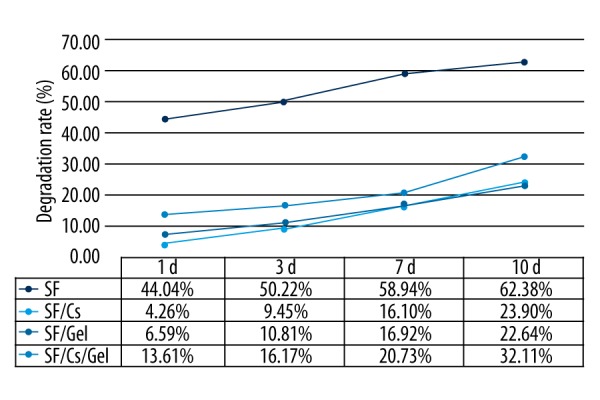
Degradation rate of the scaffolds.
Biological characterization
The biological property of the scaffolds was evaluated in vitro using MC3T3-E1 cells.
Cell adhesion rate
Analysis of cell adhesion on the scaffold was performed by cell counting using a cell counting board at 1, 3, and 6 hour (h) after cell inoculation on the pre-wetted scaffolds. The cell adhesion rate is presented in Figure 5, showing that the SF/Cs/Gel scaffold had the highest cell adhesion rate, which was from 90.00% at 1 h to 95.00% at 6 h. The SF/Cs scaffold ranked second, with a rate of 80.00% at 1 h to 85.00% at 6 h.
Figure 5.
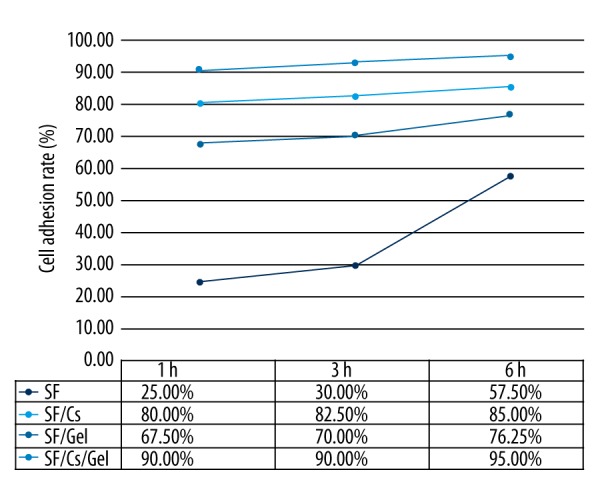
Cell adhesion rate of the scaffolds.
Cell proliferation rate
We applied a method called MTT to measure cell proliferation of the scaffolds. The result is shown in Figure 6. Cells cultured on SF/Cs/Gel scaffold had the highest OD value at all times. The SF/Gel scaffold ranked second and the SF/Cs scaffold third. We also used the t test to compare the SF/Cs/Gel group and the SF/Gel group. The P values on 4 days were 0.032, 0.013, and <0.001 and <0.001, respectively. All P values were less than 0.05, which indicated a significant difference in cell growth between the 2 scaffolds.
Figure 6.
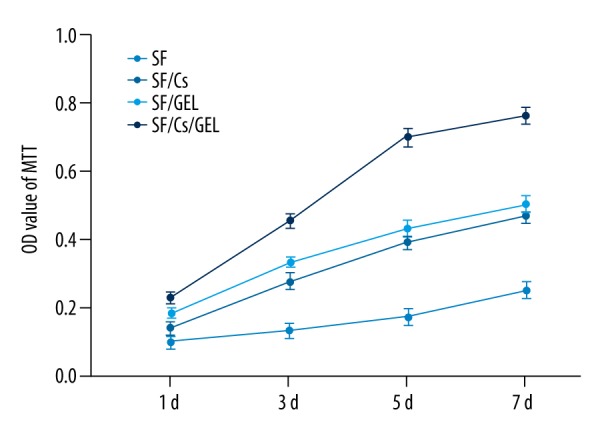
The OD value of MTT.
Cell growth and morphology
We studied cell morphology in our scaffolds through scanning electron microscopy and staining.
Scanning electron microscope
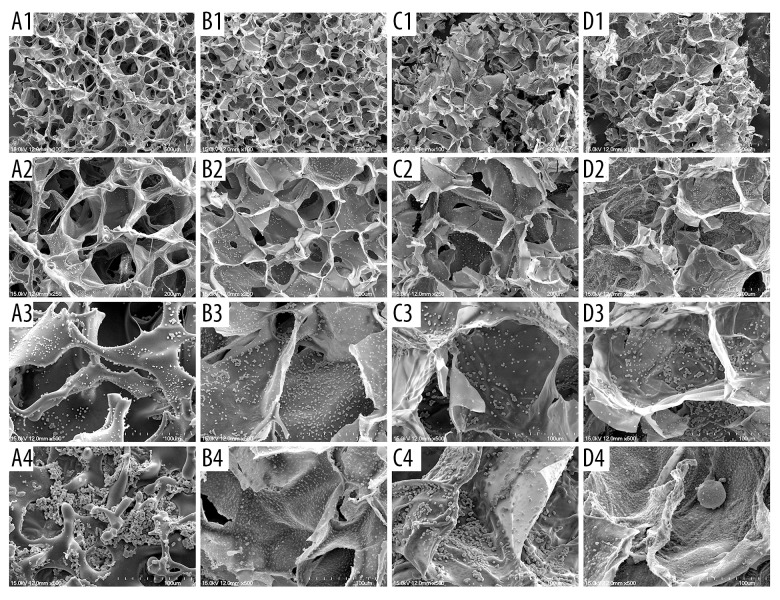
The SEM image of cell morphology on the scaffolds is presented in Figure 7. Cell morphology in the 3D scaffolds was quite different from that in the common culture dish. In the 3D culture system, cells did not adhere to the scaffold to the full extent, but maintained their original shape just like they were in vivo. With time, the cells grew more but they did not spread all over the internal surface of scaffolds; instead, they gathered and clung to each other. It is obvious that 3D scaffolds provide a much better culture system. We found that cells on the scaffolds containing Gel had better proliferation. The SF/Cs/Gel scaffold clearly cultures more cells in the same conditions.
Figure 7.
The Scanning electron microscopy image of cell morphology on the scaffolds. (A1) SF scaffold on day 3, magnified 100 times. (A2) SF scaffold on day 3, magnified 250 times. (A3) SF scaffold on day 3, magnified 500 times. (A4) SF scaffold on day 7, magnified 500 times. (B1) SF/Cs scaffold on day 3, magnified 100 times. (B2) SF/Cs scaffold on day 3, magnified 250 times. (B3) SF/Cs scaffold on day 3, magnified 500 times. (B4) SF/Cs scaffold on day 7, magnified 500 times. (C1) SF/Gel scaffold on day 3, magnified 100 times. (C2) SF/Gel scaffold on day 3, magnified 250 times. (C3) SF/Gel scaffold on day 3, magnified 500 times. (C4) SF/Gel scaffold on day 7, magnified 500 times. (D1) SF/Cs/Gel scaffold on day 3, magnified 100 times. (D2) SF/Cs/Gel scaffold on day 3, magnified 250 times. (D3) SF/Cs/Gel scaffold on day 3, magnified 500 times. (D4) SF/Cs/Gel scaffold on day 7, magnified 500 times.
Staining
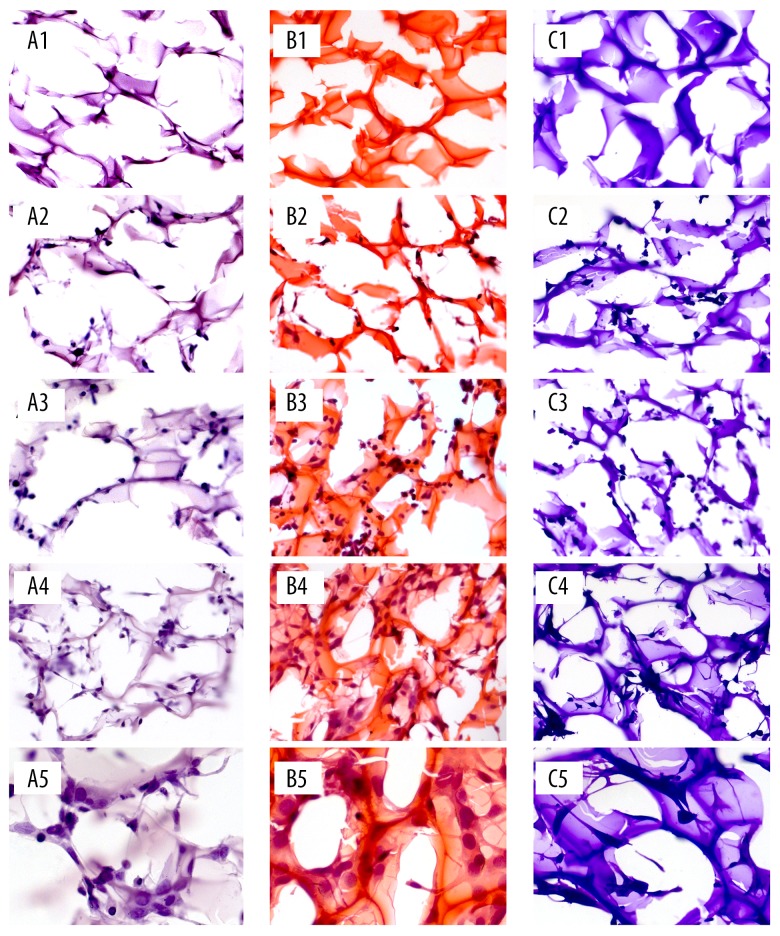
The images of hematoxylin staining, hematoxylin-eosin staining, and crystal violet staining of SF/CS/Gel scaffold is presented in Figure 8. All 3 staining methods could differentiate cells from the scaffold; however, the hematoxylin-eosin staining was the optimal one since it turned the cells into purple blue and the scaffold became orange-red, while the other 2 methods only turned cells and the scaffold into different shades of the same color. In addition, the cells on the scaffold were spherical and had a gathering tendency, which confirmed our SEM results.
Figure 8.
The images of hematoxylin staining, hematoxylin-eosin staining, and crystal violet staining of SF/CS/Gel scaffold. (A1) Without cells, stained by hematoxylin, magnified 200 times. (A2) On day 1, stained by hematoxylin, magnified 200 times. (A3) On day 4, stained by hematoxylin, magnified 200 times. (A4) On day 7, stained by hematoxylin, magnified 200 times. (A5) On day 7, stained by hematoxylin, magnified 400 times. (B1) Without cells, stained by hematoxylin-eosin. (B2) On day 1, stained by hematoxylin-eosin, magnified 200 times. (B3) On day 4, stained by hematoxylin-eosin, magnified 200 times. (B4) On day 7, stained by hematoxylin-eosin, magnified 200 times. (B5) On day 7, stained by hematoxylin-eosin, magnified 400 times. (C1) Without cells, stained by crystal violet, magnified 200 times. (C2) On day 1, stained by crystal violet, magnified 200 times. (C3) On day 4, stained by crystal violet, magnified 200 times. (C4) On day 7, stained by crystal violet, magnified 200 times. (C5) On day 7, stained by crystal violet, magnified 400 times.
Discussion
The appearance of our SF/Cs scaffold is similar to that of the SF/Cs scaffold fabricated by Deng et al. [17], which resembles the other scaffolds. Adequate pore size and porosity have a remarkable influence on cell adhesion, proliferation, and function, likely facilitated by cell spreading, increased transport of gases and nutrients to cells, and catabolite removal from cells [18]. Optimal scaffold pore size can be cell-type-specific [19]. In our study, the best scaffold for MC3T3-E1 cells was the SF/Cs/Gel scaffold, which had the biggest pore size (100–200 μm) in biological characterization. Higher-porosity scaffolds are good at cell infiltration because of enhanced cellular infiltration and mechanical interlocking [20]; however, the best porosity for bone regeneration remains unknown. Although our SF scaffold had the highest porosity, our SF/Cs/Gel scaffold, which ranked third, at 42.06%, was the optimal one for cell growth.
The water-binding ability of scaffolds is significant for tissue engineering. High water absorption rates can be attributed to the porous network structure, which affects cellular migration, proliferation, and differentiation, as well as biochemical transport and mechanical properties [21]. Good water absorption is essential for scaffolds because it guarantees the scaffold can obtain enough culture medium for cells to get ample nutrition. There have been no articles concerning which water absorption rate is optimal for cell culture [22,23].The water uptake rates of our scaffolds were all above 350%. Although the SF/Cs scaffold had the highest water uptake rate (3855.75%), the best scaffold for cell growth is our SF/Cs/Gel scaffold, which ranked third, at 537.34%. A good material for bone tissue regeneration should have a degradation rate appropriate to bone reconstruction; however, the optimal degradation rate is still unknown. From our study, we found that the SF/Cs/Gel, which was the optimal one for cell culture, ranked second in degradation rate. The specific data are 13.61% at day 1, 16.17% at day 3, 20.74% at day 7, and 32.11% at day 10.
In our biological property study, cells calculated on SF/CS/Gel scaffold presented the highest cell adhesion rate and cell proliferation rate. Our research into cell growth and morphology through SEM also showed more cells on the SF/CS/Gel scaffold. The biological characterization indicated that the SF/CS/Gel scaffold may be the best scaffold among the 4 we studied for bone regeneration. Images of the staining scaffolds and cells showed that cell morphology in the 3D scaffolds, which maintained their original shape, just like in vivo, was quite different from that in the common culture dish.
However, many questions remain unanswered. First of all, our SF/Cs/Gel scaffold had the pore size of 100 to nearly 200 μm, but we could not draw a conclusion about the optimal pore size. Further research is needed on methods to adjust pore size and decide on the best size. Our SF/Cs/Gel scaffold ranked third in water absorption rate and ranked second in degradation rate, but it possessed the best cell culture ability. No conclusion could be made concerning the relationships among water absorption rate, degradation rate, and cell growth. We only conducted the biological property study in vitro, and to certify applications in bone engineering, more in vivo research is needed.
Conclusions
We conclude that the best scaffold among the 4 we assessed for MC3T3-E1 cells is our SF/Cs/Gel scaffold, suggesting a new choice for bone regeneration to treat bone defects or fractures in clinical practice.
Footnotes
Source of support: This study was supported by the National Nature Science Foundation of China (Grant No. 8167090574)
References
- 1.Venkatesan J, Bhatnagar I, Manivasagan P, et al. Alginate composites for bone tissue engineering: A review. Int J Biol Macromol. 2015;72:269–81. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Tang D, Tare RS, Yang LY, et al. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–82. doi: 10.1016/j.biomaterials.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Li B, Xiao X, et al. Preparation and evaluation of an Arg-Gly-Asp-modified chitosan/hydroxyapatite scaffold for application in bone tissue engineering. Mol Med Rep. 2015;12(5):7263–70. doi: 10.3892/mmr.2015.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salerno A, Zeppetelli S, Di ME, et al. Processing/structure/property relationship of multi-scaled PCL and PCL-HA composite scaffolds prepared via gas foaming and NaCl reverse templating. Biotechnol Bioeng. 2011;108(4):963–76. doi: 10.1002/bit.23018. [DOI] [PubMed] [Google Scholar]
- 5.Scaglione S, Lazzarini E, Ilengo C, Quarto R. A composite material model for improved bone formation. J Tissue Eng Regen Med. 2010;4(7):505–13. doi: 10.1002/term.265. [DOI] [PubMed] [Google Scholar]
- 6.Xie J, Peng C, Zhao Q, et al. Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater. 2016;29:365–79. doi: 10.1016/j.actbio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Lin L, Gao H, Dong Y. Bone regeneration using a freeze-dried 3D gradient-structured scaffold incorporating OIC-A006-loaded PLGA microspheres based on β-TCP/PLGA. J Mater Sci Mater Med. 2015;26(1):5327. doi: 10.1007/s10856-014-5327-9. [DOI] [PubMed] [Google Scholar]
- 8.Melke J, Midha S, Ghosh S, et al. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16. doi: 10.1016/j.actbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Kim IY, Seo SJ, Moon HS, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26(1):1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24(13):2339–49. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 11.Islam MM, Khan MA, Rahman MM. Preparation of gelatin based porous biocomposite for bone tissue engineering and evaluation of gamma irradiation effect on its properties. Mater Sci Eng C Mater Biol Appl. 2015;49:648–55. doi: 10.1016/j.msec.2015.01.066. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim DK, Lee OJ, et al. Osteoinductive silk fibroin/titanium dioxide/hydroxyapatite hybrid scaffold for bone tissue engineering. Int J Biol Macromol. 2016;82:160–67. doi: 10.1016/j.ijbiomac.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Maji K, Dasgupta S, Kundu B, Bissoyi A. Development of gelatin-chitosan-hydroxyapatite based bioactive bone scaffold with controlled pore size and mechanical strength. J Biomater Sci Polym Ed. 2015;26(16):1190–209. doi: 10.1080/09205063.2015.1082809. [DOI] [PubMed] [Google Scholar]
- 14.Rajzer I, Menaszek E, Kwiatkowski R, et al. Electrospun gelatin/poly(ɛ-caprolactone) fibrous scaffold modified with calcium phosphate for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;44:183–90. doi: 10.1016/j.msec.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Kim UJ, Park J, Kim HJ, et al. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26(15):2775–85. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 16.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718–26. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, She R, Huang W, et al. A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J Mater Sci Mater Med. 2013;24(8):2037–46. doi: 10.1007/s10856-013-4944-z. [DOI] [PubMed] [Google Scholar]
- 18.Nava MM, Draghi L, Giordano C, Pietrabissa R. The effect of scaffold pore size in cartilage tissue engineering. J Appl Biomater Funct Mater. 2016;14(3):e223–29. doi: 10.5301/jabfm.5000302. [DOI] [PubMed] [Google Scholar]
- 19.Murphy CM, Duffy GP, Schindeler A, O’brien FJ. Effect of collagen-glycosaminoglycan scaffold pore size on matrix mineralization and cellular behavior in different cell types. J Biomed Mater Res A. 2016;104(1):291–304. doi: 10.1002/jbm.a.35567. [DOI] [PubMed] [Google Scholar]
- 20.Ionescu LC, Mauck RL. Porosity and cell preseeding influence electrospun scaffold maturation and meniscus integration in vitro. Tissue Eng Part A. 2013;19(3–4):538–47. doi: 10.1089/ten.tea.2012.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huri PY, Ozilgen BA, Hutton DL, Grayson WL. Scaffold pore size modulates in vitro osteogenesis of human adipose-derived stem/stromal cells. Biomed Mater. 2014;9(4):045003. doi: 10.1088/1748-6041/9/4/045003. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yang Q, Cheng N, et al. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater Sci Eng C Mater Biol Appl. 2016;61:705–11. doi: 10.1016/j.msec.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 23.Thadavirul N, Pavasant P, Supaphol P. Improvement of dual-leached polycaprolactone porous scaffolds by incorporating with hydroxyapatite for bone tissue regeneration. J Biomater Sci Polym Ed. 2014;25(17):1986–2008. doi: 10.1080/09205063.2014.966800. [DOI] [PubMed] [Google Scholar]