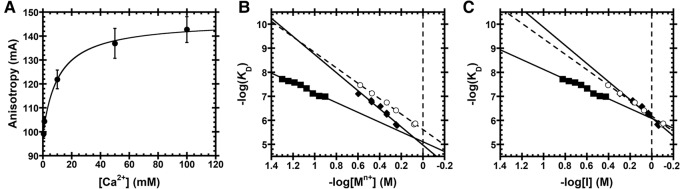
FIGURE 3.
Cation (Mn+) dependence of dissociation constants for AtPRORP1 binding to B. subtilis fluorescein-labeled pre-tRNAAsp. (A) Anisotropy of Bacillus subtilis pre-tRNAAsp in the absence of PRORP is dependent on CaCl2 concentration. Data reported as the mean and standard deviation of four independent experiments. A hyperbola (Equation 1, Materials and Methods) was fit to the data (KD,app = 11±3 mM). (B) Equation 4 (Materials and Methods) was fit to the data with the dsDNA ϕNa = 0.88 or φMg = 0.47. Data include the dependence of AtPRORP1 affinity on NaCl [♦, Z = 4.3 ± 0.3, log(K0) = 5.0 ± 0.1], Na2SO4 [◯, Z = 3.6 ± 0.1, log(K0) = 5.62 ± 0.03], and CaCl2 [▪, Z = 4.3 ± 0.3, log(K0) = 5.1 ± 0.2]. The slope of the line (Zϕ or Zφ) reports on the apparent number of ionic interactions made to substrate phosphodiester bonds, while the intercept [log(K0)] reports on the nonionic contributions to affinity. (C) Ionic strength (I) dependence of AtPRORP1 binding to pre-tRNAAsp, plotted as the −log(KD) versus −log[I]. Equation 4 (Materials and Methods) was fit to the data with ϕNa = 0.88 or φCa = 0.47. Data include AtPRORP1 binding in NaCl [♦; log(K0) = 6.11 ± 0.04], Na2SO4 [◯; log(K0) = 6.18 ± 0.02], and CaCl2 [▪; log(K0) = 6.08 ± 0.09].