Abstract
Objective:
The present study aimed to evaluate left ventricular (LV) systolic function in patients with iron deficiency anemia (IDA) by 3-dimensional speckle-tracking echocardiography (3DSTE).
Methods:
Participants were grouped by hemoglobin (Hb) levels in order to study the effect of anemia on cardiac function. Group A included 40 healthy volunteers. Eighty-three patients who were diagnosed with IDA were divided into 2 groups according to the Hb level. Group B (Hb 9 g/dL) included 44 patients, while group C (Hb 6–9 g/dL) included 39 patients. Left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left ventricular mass index (LVMI), and left ventricular ejection fraction (LVEF) were calculated by real-time 3-dimensional echocardiography (RT3D). Left ventricular global longitudinal strain (GLS), global area strain (GAS), global radial strain (GRS), and global circumferential strain (GCS) were obtained by 3DSTE.
Results:
LVMI, LVEDV and LVESV of group C increased and GCS, GRS, GLS, and GAS of group C decreased compared with those of groups A and B (all p<0.05). GAS and GLS decreased significantly compared with other parameters (both p<0.01).
Conclusion:
LV remodeling and LV systolic dysfunction occurred in patients when the hemoglobin level was in the range of 6–9 g/dL. 3DSTE can evaluate LV systolic function in patients with IDA, and GAS and GLS are more sensitive than other parameters.
Keywords: 3-dimensional tracking echocardiography, iron deficiency anemia, left ventricular systolic function
Introduction
Anemia is one of the most common diseases in the world. The global prevalence of anemia was 32.9% at the last count in 2010 (1). Iron deficiency is a chief cause of anemia, although there are numerous other reasons. Iron deficiency affects multiple organ systems such as the nervous system, circulatory system, and immune system (2–5). Moreover, anemia is considered to be an independent risk factor for cardiovascular disease (CVD) outcomes in the general population (6, 7). Anemia is correlated with increased morbidity and mortality in patients with heart failure (8). A previous study has shown that cardiac hypertrophy occurs in dogs with chronic severe anemia by repeated venesections (9). Another study (10) revealed no consistent change in circulation at a hemoglobin (Hb) level above 7 g/dL. However, few studies have evaluated whether mild and mode-rate anemia influences the heart. Therefore, clinical research on the effect of anemia on the cardiac structure and function would be very meaningful. Patients with iron deficiency anemia (IDA) can present with symptoms that are associated with all anemias, including specific symptoms. Pallor of the skin, conjunctivae, and nail beds are common. Other symptoms and signs result from hypoxic functioning. These include fatigue, exertional dyspnea progressing to breathlessness at rest, vertigo, syncope, headache, tachycardia, and a cardiac systolic flow murmur (11).
Three-dimensional speckle-tracking echocardiography (3DSTE) is a recently introduced technology, which has been proved to be a reliable quantitative tool for evaluating left ventricular (LV) myocardial function (12). It is a comparatively angle-independent technology that can measure global and regional strain and velocity in longitudinal, radial, and circumferential directions (13, 14). 3DSTE has been already used to assess coronary heart disease (15), hypertrophic cardiomyopathy (16), and LV mechanical dyssynchrony (17). The aim of the present study was to explore the early changes in LV systolic function in patients with anemia in varying degrees.
Methods
Informed consent
The design proposal and manners of date collection and analysis were approved by the Ethics Committee. All the subjects gave their written informed consent after receiving a detailed explanation of the study protocol and of the potential risks related to the procedures adopted in the study.
Diagnostic standard of anemia and grouping principles
Diagnostic criteria of IDA include the following: 1. Hb values: adult men, <13 g/dL; adult women, <12 g/dL; and adult pregnant women, <11 g/dL. 2. Microcytic hypochromic anemia: mean corpuscular volume (MCV) <80 fL; mean corpuscular Hb (MCH) level <27 pg; and MHC concentration (MCHC) <32%. 3. Serum iron concentration <8.95 µmol/L, serum ferritin concentration <12 µg/L, total iron binding capacity >64.44 µmol/L, serum transferrin saturation <15%, and serum transferring receptor concentration >8 mg/L. 4. Free erythrocyte protoporphyrin (FEP) concentration >0.9 µmol/L, zinc protoporphyrin (ZPP) concentration >0.96 µmol/L, and FEP/Hb >4.5 µg/g. There were 3 groups of Chinese populations in the present study based on Hb levels according to Internal Medicine as follows: Control group Hb levels: adult men, Hb ≥13 g/dL; adult women, Hb ≥12 g/dL. Mild anemia Hb levels: adult men, 9 g/dL < Hb < 13 g/dL; adult women, 9 g/dL < Hb < 12 g/dL. Moderate anemia Hb levels: 6 g/dL ≤ Hb ≤ 9 g/dL.
Study design and population
From November 2014 to July 2016, a total of 83 patients with IDA (ratio of females to males: 6:1; age, 21–66 years; disease duration, 7 months–15 years) and 40 age-and gender-matched healthy volunteers (age, 22–65 years; mean age, 47.4±13.7 years) were studied. All of them had been diagnosed with IDA for more than 6 months, and their Hb level remained stable. Four patients had taken iron supplements regularly but were still suffering from mild anemia. Five patients received blood transfusions, but repeated examinations showed no obvious change in long-term anemia. The exclusion criteria included coronary heart disease, hypertension, chronic obstructive pulmonary disease, heart valve disease, congenital heart disease, continuous arrhythmia, hyperlipemia, diabetes mellitus, rheumatic heart disease, chronic kidney dysfunction, and chronic inflammatory disease. Forty-seven patients had IDA caused by gynecological diseases such as uterine fibroid, endometriosis, and menorrhagia. Twenty-seven patients had IDA caused by digestive system diseases and procedures such as subtotal gastrectomy for gastroduodenal ulcer, intestinal polyp, hemorrhoids, and anal fissure. The reasons were unknown in 9 patients. These patients were divided into 2 groups according to the Hb level: group B (44 patients, Hb >9 g/dL; age, 25–66 years; mean age, 46.3±13.3 years; disease duration, 7 months–15 years; mean course of disease, 6.0±3.7 years) and group C (39 patients, Hb, 6–9 g/dL; age, 21–62 years; mean age, 48.2±16.1 years; disease duration, 8 months–14 years; mean course of disease, 6.5±3.2 years). None of the participants had been diagnosed with cardiovascular disorders, and findings of comprehensive physical examinations were normal.
Physical examination
Heights and weights of all the participants were measured. The body surface area and body mass index (BMI) of every participant were calculated. Blood pressures were measured for 3 times interval to 10 min at a resting state for 15 min. All the participants had accepted to undergo routine physical examination, other examinations including serum lipid examination, blood glucose examination, electrocardiogram examination, liver function tests, renal function tests, thyroid function tests, coronary angiography, and pulmonary function test.
Conventional echocardiography image acquisition and analysis
The subjects rested in the left lateral decubitus position for the electrocardiogram recording, which was performed using a Vivid E9 commercial ultrasound scanner (E9, GE Health Care, American, Vingmed Ultrasound AS) with phased-array transducers (M5S-D and 4V-D). 2D images were obtained from apical 4-chamber and long-axis views. The gray scale was adjusted and focused to optimize for LV evaluation. Three consecutive cardiac cycles were stored for each view during quiet respiration. LV end-diastolic diameter (LVDd), LV end-systolic diameter (LVDs), interventricular septum thickness diastolic (IVSTd), posterior wall thickness diastolic (PWTd), and ratio of early-to-late peak velocities (E/A) were calculated.
3DSTE image acquisition and analysis
3DSTE data acquisition was performed with a fully sampled matrix-array transducer. One cardiac cycle was recorded with full-volume scan during end-expiratory apnoea, and the transducer was kept in a stable position at a frame rate of 25–50 frames/s. The view was kept in an optimal temporal and spatial resolution, and it included the whole LV cavity, myocardium, and epicardium. All images were digitally stored for offline analysis [EchoPAC software worksation (version BT8, 4D Auto LVQ; GE Vingmed Ultrasound AS)]. The RT3D and 3DSTE data were analyzed by the software, which allowed manual adjustment for detection of the region of interest (ROI) at the myocardium and epicardium border. Tracing of ROI allowed to obtain the LV ejection fraction (LVEF), LV end-diastolic volume (LVEDV) and end-systolic volume (LVESV), and LV mass index (LVMI). The peak systolic strain values of all the 17 myocardial segments from LV were shown as bull’s eye figures. LV global longitudinal strain (GLS), global area strain (GAS), global radial strain (GRS), and global circumferential strain (GCS) were calculated as weighted averages of the regional values from the 17 myocardial segments. The inadequately tracked segments (less than 3) were excluded, and the strain values were not included in the calculations of global strain values.
Statistical analysis
Statistical analysis was performed by SPSS 17.0 package (SPSS 17.0, Inc, Chicago, IL, USA). Data are presented as mean values±standard deviations (SDs). A normal distribution of our data was shown using the normality test and distribution curve. One-way ANOVA was performed to test for statistically significant differences among the 3 groups. Continuous data were compared between different groups using unpaired Student’s–Newman–Keuls tests and presented as mean±SD, with p<0.05 being statistically significant. The reproducibility and repeatability of the global LV strain were measured in our study in 25 randomly selected patients in each group by 2 observers. A second observer who was blinded to the results of the first investigator repeated the measurements to assess interobserver variability. Intraobserver variability and interobserver variability were assessed using the intraclass correlation coefficient (ICC).
Results
Demographics of the study population of IDA patients and control subjects are summarized in Table 1. There was no significant difference among the 3 groups in terms of age, gender, duration of IDA, heart rate, body mass index, blood glucose, and systolic or diastolic blood pressure.
Table 1.
Demographics of the study population
| Demographic characteristics/Risk factors | Group A (n=40) | Group B (n=44) | Group C (n=39) |
|---|---|---|---|
| Male-to-female ratio | 1: 7 | 3:19 | 5:34 |
| Age, years | 47.4±13.7 | 46.3±13.3 | 48.2±16.1 |
| Duration of IDA, years | 0 | 6.0±3.7 | 6.5±3.2 |
| Heart rate, rates/min | 71±11 | 73±9 | 73±11 |
| Body mass index, kg/m2 | 22.1±2.4 | 22.7±2.5 | 22.3±2.4 |
| Systolic arterial pressure, mm Hg | 120±10 | 123±12 | 121±14 |
| Diastolic arterial pressure, mm Hg | 72±7 | 74±9 | 74±10 |
Data are expressed as mean±SD except for the female-to–male ratio. All P>0.05
The conventional echocardiographic measurements are shown in Table 2. LVDd, LVDs, IVSTd, and PWTd showed no differences among the three groups (all p>0.05); a similar result was also observed with regard to the E/A ratio between groups A and B. Nevertheless, compared with groups A and B, the E/A ratio of group C decreased remarkably (p<0.05).
Table 2.
Two-dimensional ultrasound and Doppler flow image parameters of the study population
| Variables | Group A (n=40) | Group B (n=44) | Group C (n=39) |
|---|---|---|---|
| LVDd, mm | 42.8±2.39 | 43.6±2.66 | 44.8±2.79 |
| LVDs, mm | 28.2±2.64 | 29.3±2.32 | 30.7±2.68 |
| IVSTd, mm | 8.8±0.69 | 9.0±0.59 | 9.1±0.67 |
| PWTd, mm | 8.7±0.67 | 8.9±0.52 | 8.9±0.58 |
| E/A ratio | 1.1±0.39 | 1.1±0.34 | 0.8±0.30* |
Compared with groups A and B;
P<0.05.
Data are expressed as mean±SD. E/A ratio, the ratio of the early (E) to late (A) ventricular filling velocities; IVSTd – interventricular septum thickness diastolic; LVDd - left ventricular end-diastolic diameter; LVDs - left ventricular end-systolic diameter; PWTd - left ventricular posterior wall diastolic thickness
RT3D and 3DSTE parameters of the 3 groups are shown in Table 3. With regard to RT3D parameters, there were no differences between groups A and B in terms of LVMI, LVEDV, and LVESV (all p>0.05). Compared with groups A and B, group C showed a signi-ficant decrease in LVMI, LVEDV, and LVESV (all p<0.05). However, LVEF showed no significant difference among the 3 groups. With regard to 3DSTE parameters, there was no significant difference between groups A and B in terms of GLS, GAS, GRS, and GCS (all p>0.05). GLS, GAS, GRS, and GCS of group C showed a remarkable decline, and the strain curve amplitude was lower than that of groups A and B (all p<0.05). It is noteworthy that GAS and GLS decreased significantly compared with other parameters (both p<0.01). The longitudinal, circumferential, area, and radial strains of the 3 groups are shown in Figure 1.
Table 3.
RT3D and 3DSTE parameters of the study population
| Variables | Group A (n=40) | Group B (n=44) | Group C (n=39) |
|---|---|---|---|
| 3DRT | |||
| LVMI, g/m2 | 72.5±8.2 | 73.8±7.9 | 77.4±6.8†§ |
| LVEF, % | 64.9±4.3 | 65.4±4.2 | 63.7±4.8 |
| LVEDV, mL | 94.9±31.7 | 97.2±29.4 | 115.0±27.4†§ |
| LVESV, mL | 35.7±12.3 | 36.9±10.1 | 40.2±9.9†§ |
| 3DSTE | |||
| GLS, % | -21.4±2.7 | -20.8±2.6 | -18.3±1.8‡|| |
| GRS, % | 53.4±11.0 | 53.1±10.6 | 50.0±9.1†§ |
| GCS, % | -19.8±4.2 | -19.6±4.6 | -18.1±3.7†§ |
| GAS, % | -34.2±4.3 | -33.8±4.1 | -31.1±2.9‡|| |
Compared with group A, #: 0.01<P<0.05;
: P<0.01; Compared with group B,
: 0.01<P<0.05;
: P<0.01.
Data are expressed as mean±SD. GAS - global area strain; GCS – global circumferential strain; GLS - global longitudinal strain; GRS - global radial strain; LVEDV - left ventricular end-diastolic volume; LVEF - left ventricular ejection fraction; LVESV - left ventricular end-systolic volume; LVMI - left ventricular mass index
Figure 1.
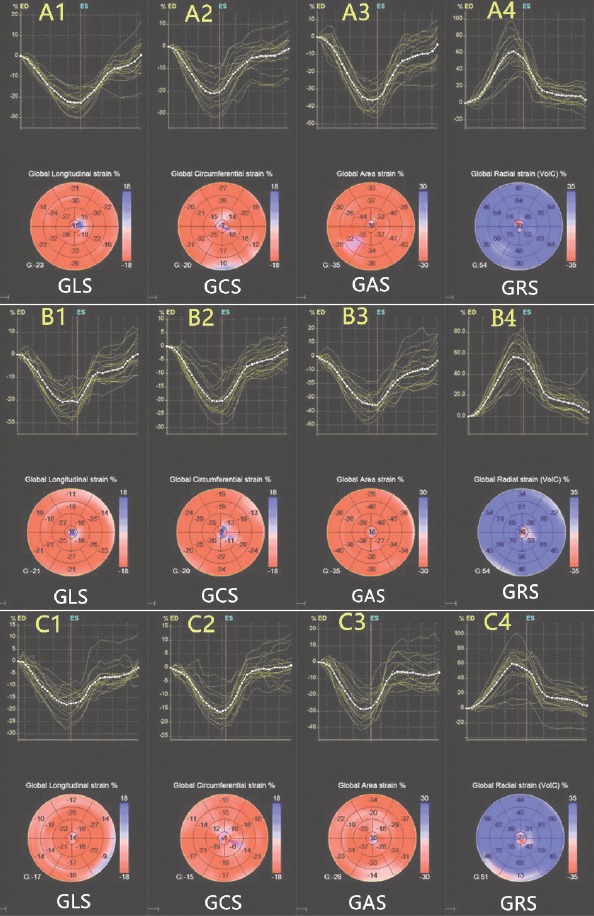
Longitudinal, circumferential, area, and radial strains of the 3 groups are generated and presented in both (regional and average) strain curves and color-coded, 17-segment bull’s eye plots by 3DSTE. Colored lines refer to the regional strain, and the white dotted line refers to the global strain. A1: healthy control group longitudinal strain; A2: healthy control group circumferential strain; A3: healthy control group area strain; A4: healthy control group radial strain; B1: group B longitudinal strain; B2: group B circumferential strain; B3: group B area strain; B4: group B radial strain; C1: group C longitudinal strain; C2: group C circumferential strain; C3: group C area strain; C4: group C radial strain.
C1, C2, C3, and C4 showed reduced global strain values compared with groups A and B. Lower magnitude of strain reflects decreased cardiac function
Intra-and interobserver variabilities in the 3DSTE para-meters are shown in Table 4. The inter-and intraobserver results revealed good reproducibility and small variability in the evaluation of LV global strain values by 3DSTE in patients with IDA.
Table 4.
Inter- and intraobserver analyses for global strain and twist values
| Intraobserver variability | Interobserver variability, Rho (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | R | Bias (%) | LOA (%) | ICC | R | Bias (%) | LOA (%) | ICC |
| GAS (%) | 0.86 | 1.25 | -4.32~2.66 | 0.921 | 0.84 | 1.21 | -5.05~4.78 | 0.891 |
| GCS (%) | 0.80 | 2.23 | -6.37~5.29 | 0.855 | 0.78 | 2.83 | -8.06~3.22 | 0.812 |
| GLS (%) | 0.91 | 0.68 | -1.15~2.01 | 0.971 | 0.93 | 0.55 | -0.63~1.15 | 0.987 |
| GRS (%) | 0.83 | 1.95 | -6.05~4.19 | 0.870 | 0.80 | 2.11 | -6.45~4.88 | 0.846 |
GAS - global area strain; GCS - global circumferential strain; GLS - global longitudinal strain; GRS - global radial strain; ICC - intraclass correlation coefficient; LOA - limit of agreement; R - coefficient of determination
Discussion
We inspected the LV structure and systolic function in patients with IDA by 3DSTE, including the LV volume, LVMI, and strain components. This approach confirmed the increase in the LV mass and LV volume and decrease in strain values. Similarly, we also found that the exceptions involved GLS, GRS, GCS, and GAS in patients with IDA when the Hb level was in the range of 6–9 g/dL even without LV hypertrophy or LV enlargement with normal EF. Remarkably, GAS and GLS were more sensitive in terms of the evaluation of LV systolic dysfunction. None of the patients with IDA in our study experienced heart failure.
Patients with IDA showed varying degrees of pallor of the skin, conjunctivae, and nail beds. Most patients with moderate anemia narrated that they often experienced fatigue, exertional dyspnea progressing to breathlessness at rest, vertigo, syncope, headache, and tachycardia after physical activity. Patients with mild anemia narrated that they could hardly feel uncomfortable in a resting state. However, other symptoms and signs resulting from hypoxic functioning occurred after physical activity, such as fatigue, exertional dyspnoea progressing to breathlessness at rest, and vertigo. In previously published studies (18, 19), cardiac functional damage was determined by X-ray and conventional echocardiography in patients with anemia; it was found that cardiac enlargement, LV hypertrophy, and heart dysfunction are common in patients with chronic severe anemia. It is difficult to diagnose cardiac damage at an early stage. 3DSTE is a promising technique; its accuracy and reliability have been shown to closely correspond to those of MRI (20, 21). It has been widely used in clinical research and has been found to be a useful method to assess the myocardial structure and cardiac systolic function (22). In our study, 3DSTE showed more reliable results, and was verified to reflect myocardial contractility, and provided liable and sensitive parameters for the early detection of myocardial injury.
In our study, we found all parameters showed no significant difference between groups A and B; the main cause could be that the effect on cardiac function was slightly when the Hb level of the patients with IDA was above 9 g/dL and the heart exhibited adaptive potential remodeling via the Frank–Starling mechanism. Another study (23) showed that when the Hb level was below 7 g/dL, the cardiac output began to increase at rest, while the arteriovenous oxygen difference and peripheral resistance dec-reased. In the present study, LVMI, LVEDV, and LVESV in group C were significantly increased compared with those in groups A and B. The results were consistent with a previous report that a low Hb level can cause heart enlargement and the LV mass and LV filling pressure increases in patients with IDA (24). This is mainly related to a persistently hyperdynamic circulatory state in patients with IDA, which is associated with remodeling of the myocytes and vasculature (25). Our study showed that GLS, GCS, GAS, and GRS values were lower in group C than the other 2 groups; all of them showed a significant difference. Aessopos et al. (25) reached a similar conclusion: anemia in the elderly caused light of the marginally compromised cardiac function.
Notably, the results of the present study showed an evidence of LV contractile dysfunction, GAS and GLS have been shown to be ideal parameters in many studies (26, 27). Our findings were in accordance with this. LV myocardial fiber construction is considered to be structured by a single muscular band that 2 two spirals in the space of the basal loop (BL) and the apical loop (AL). The function of BL fibers is to run in a transversal plane of ventricles. In AL, the fibers include the descendent segment (DS) and ascendant segment (AS) that predominantly run in the vertical direction (28). Longitudinal fibers are essential for normal systolic function in terms of both the extent and timing of shortening and thickening (29, 30), and consecutive contraction waves preferably follow the longitudinal axes of myocardial fibers (28). Compared with circular motion and shortening of the ventricular longitudinal axis, longitudinal motion has stronger contractility, higher stress, and greater oxygen demand, making them more vulnerable to ischemia and hypoxia (31, 32). GAS is a new 3DSTE index that represents the endocardial area deformation, mainly determined by longitudinal and circumferential motions. It enables a relatively operator-independent quantitative evaluation of global and regional LV function (29).
Clinical implications
In the present study, we found that 3DSTE is an effective approach to discover LV dysfunction in patients with IDA. It is extremely helpful for the early detection of abnormal myocardial function. Furthermore, it could help indicate the clinical therapy, reduce hospital stay, improve the quality of life, and decrease the risk of morbidity and mortality. Further, our findings are likely to have clinical implications for better management of such patients.
Study limitations
Our study still had several limitations. First, most of our study objects were females and adults, and our results may not be relevant to males or to other age groups. Second, the treatment of IDA and Hb fluctuation may influence the grouping and development of disease (33). Third, there was no postmortem examination in our study, and although the ultrasound technique is a mature technology to diagnose heart disease, biopsy still remains the diagnostic gold standard technique.
Conclusion
We study found that patients with Hb levels below 9 g/dL showed a change in LV structure and function by 3DSTE. 3DSTE is a more dependable technology to reflect LV systolic dysfunction of the myocardium and provides sensitive parameters for the early detection of myocardial injury. Moreover, GAS and GLS are more sensitive than other parameters.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – G.L.; Design – Q.Z., J.S., Y.L.; Supervision – G.L.; Funding – Q.Z., J.S., Y.L., R.L., B.T.; Materials – Q.Z., J.S., Y.L., R.L., B.T.; Data collection &/or processing – Q.Z., J.S., Y.L., Analysis &/or interpretation – G.L., Q.Z.; Literature search – G.L.; Writing – G.L., Q.Z.; Critical review – G.L., R.L., B.T.
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:34–43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5:293–9. doi: 10.1016/j.sleep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Naito Y, Tsujino T, Matsumoto M, Sakoda T, Ohyanagi M, Masuyama T. Adaptive response of the heart to long-term anemia induced by iron deficiency. Am J Physiol Heart Circ Physiol. 2009;296:585–93. doi: 10.1152/ajpheart.00463.2008. [DOI] [PubMed] [Google Scholar]
- 5.Das I, Saha K, Mukhopadhyay D, Roy S, Raychaudhuri G, Chatterjee M, et al. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children:A case control study. J Nat Sci Biol Med. 2014;5:158–63. doi: 10.4103/0976-9668.127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, et al. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 7.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–34. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JE. Emerging role of anemia in heart failure. Am J Cardiol. 2007;99(6B):15D–20D. doi: 10.1016/j.amjcard.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Paplanus SH, Zbar MJ, Hays JW. Cardiac hypertrophy as a manifestation of chronic aniemia. Am J Pathol. 1958;34:149–59. [PMC free article] [PubMed] [Google Scholar]
- 10.Brannon ES, Merrill AJ, Warren JV, Stead EA. The cardiac output in patients with chronic anemia as measured by the technique of right atrial catheterization. J Clin Invest. 1945;24:332–6. doi: 10.1172/JCI101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–16. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 12.Altman M, Bergerot C, Aussoleil A, Davidsen ES, Sibellas F, Ovize M, et al. Assessment of left ventricular systolic function by deformation imaging derived from speckle tracking:a comparison between 2D and 3D echo modalities. Eur Heart J Cardiovasc Imaging. 2014;15:316–23. doi: 10.1093/ehjci/jet103. [DOI] [PubMed] [Google Scholar]
- 13.Biswas M, Sudhakar S, Nanda NC, Buckberg G, Pradhan M, Roomi AU, et al. Two- and three-dimensional speckle tracking echocardiography:clinical applications and future directions. Echocardiography. 2013;30:88–105. doi: 10.1111/echo.12079. [DOI] [PubMed] [Google Scholar]
- 14.Muraru D, Cucchini U, Mihăilă S, Miglioranza MH, Aruta P, Cavalli G, et al. Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects:reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr. 2014;27:858–71. doi: 10.1016/j.echo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Hayat D, Kloeckner M, Nahum J, Ecochard-Dugelay E, Dubois-Randé JL, Jean-François D, et al. Comparison of real-time three-dimensional speckle tracking to magnetic resonance imaging in patients with coronary heart disease. Am J Cardiol. 2012;109:180–6. doi: 10.1016/j.amjcard.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Voilliot D, Huttin O, Hammache N, Filippetti L, Vaugrenard T, Aliot E, et al. Impact of global and segmental hypertrophy on two-dimensional strain derived from three-dimensional echocardiography in hypertrophic cardiomyopathy:comparison with healthy subjects. J Am Soc Echocardiogr. 2015;28:1093–102. doi: 10.1016/j.echo.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Thebault C, Donal E, Bernard A, Moreau O, Schnell F, Mabo P, et al. Real-time three-dimensional speckle tracking echocardiography:a novel technique to quantify global left ventricular mechanical dyssynchrony. Eur J Echocardiogr. 2011;12:26–32. doi: 10.1093/ejechocard/jeq095. [DOI] [PubMed] [Google Scholar]
- 18.Bahl VK, Malhotra OP, Kumar D, Agarwal R, Goswami KC, Bajaj R, et al. Noninvasive assessment of systolic and diastolic left ventricular function in patients with chronic severe anemia:a combined M-mode, two-dimensional, and Doppler echocardiographic study. Am Heart J. 1992;124:1516–23. doi: 10.1016/0002-8703(92)90066-5. [DOI] [PubMed] [Google Scholar]
- 19.Sanghvi LM, Misra SN, Banerjee K. Cardiac enlargement in chronic severe anemia. Circulation. 1960;22:412–8. doi: 10.1161/01.cir.22.3.412. [DOI] [PubMed] [Google Scholar]
- 20.Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, et al. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking:comparison with MRI. Eur Heart J. 2009;30:1565–73. doi: 10.1093/eurheartj/ehp187. [DOI] [PubMed] [Google Scholar]
- 21.Kleijn SA, Brouwer WP, Aly MF, Rüssel IK, de Roest GJ, Beek AM, et al. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging. 2012;13:834–9. doi: 10.1093/ehjci/jes030. [DOI] [PubMed] [Google Scholar]
- 22.Reant P, Barbot L, Touche C, Dijos M, Arsac F, Pillois X, et al. Evaluation of global left ventricular systolic function using three-dimensional echocardiography speckle-tracking strain parameters. J Am Soc Echocardiogr. 2012;25:68–79. doi: 10.1016/j.echo.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Brannon ES, Merrill AJ, Warren JV, Stead EA. The cardiac output in patients with chronic anemia as measured by the technique of right atrial catheterization. J Clin Invest. 1945;24:332–6. doi: 10.1172/JCI101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho IJ, Mun YC, Kwon KH, Shin GJ. Effect of anemia correction on left ventricular structure and filling pressure in anemic patients without overt heart disease. Korean J Intern Med. 2014;29:445–53. doi: 10.3904/kjim.2014.29.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aessopos A, Deftereos S, Farmakis D, Corovesis C, Tassiopoulos S, Tsironi M, et al. Cardiovascular adaptation to chronic anemia in the elderly:an echocardiographic study. Clin Invest Med. 2004;27:265–73. [PubMed] [Google Scholar]
- 26.Reant P, Barbot L, Touche C, Dijos M, Arsac F, Pillois X, et al. Evaluation of global left ventricular systolic function using three-dimensional echocardiography speckle-tracking strain parameters. J Am Soc Echocardiogr. 2012;25:68–79. doi: 10.1016/j.echo.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Gao Y, Tan K, Xia H, Li P. Assessment of left ventricular function by three-dimensional speckle-tracking echocardiography in well-treated type 2 diabetes patients with or without hypertension. J Clin Ultrasound. 2015;43:502–11. doi: 10.1002/jcu.22268. [DOI] [PubMed] [Google Scholar]
- 28.Torrent-Guasp F, Kocica MJ, Corno A, Komeda M, Cox J, Flotats A, et al. Systolic ventricular filling. Eur J Cardiothorac Surg. 2004;25:376–86. doi: 10.1016/j.ejcts.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Saccheri MC, Cianciulli TF, Lax JA, Gagliardi JA, Cáceres GL, Quarin AE, et al. Two-dimensional speckle tracking echocardiography for early detection of myocardial damage in young patients with Fabry disease. Echocardiography. 2013;30:1069–77. doi: 10.1111/echo.12216. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Wei X, Liang Y, Liu M, Li C, Tang H. Differential changes of left ventricular myocardial deformation in diabetic patients with controlled and uncontrolled blood glucose:a three-dimensional speckle-tracking echocardiography–based study. J Am Soc Echocardiogr. 2013;26:499–506. doi: 10.1016/j.echo.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Breaker SJ. The importance of long-axis ventricular function. Heart. 2000;84:577–9. doi: 10.1136/heart.84.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henein MY, Gibson DG. Normal long-axis function. Heart. 1999;81:111–3. doi: 10.1136/hrt.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegde N, Rich MW, Gayomali C. The cardiomyopathy of iron deficiency. Tex Heart Inst J. 2006;33:340–4. [PMC free article] [PubMed] [Google Scholar]


