Abstract
Objective
Research which indicates that adverse experiences influence hypothalamic-pituitary-adrenal (HPA) axis functioning illustrates the social environment “getting under the skin”. The present study extended this literature by examining whether positive social forces within the caregiving environment can also impact cortisol functioning.
Method
We conducted a prospective investigation of over 300 youth, half of whom were White and half were Black. Attachment, bonding and parental rewards for positive behaviors were observed or reported by the youth as an 8th-grader. Twelve repeated measures of salivary cortisol were examined six years later when youth were young adults (mean age 20). Race differences were explored.
Results
Stronger attachment, bonding and teen-reported positive parenting were predictive of high waking cortisol and steeper diurnal slopes six years later. This effect was nonlinear and additive, such that youth whose social contexts were characterized by the strongest attachment, bonding and rewarding parental relationships had the highest waking cortisol. When effects were moderated by race, findings were such that links of positive parenting with HPA functioning were more consistent for White than Black youth.
Conclusions
Findings suggest that positive aspects of the caregiving environment can also “get under the skin” and these effects are additive across a range of caregiving indices. These findings dovetail with an emerging literature on the powerful role of social support for shaping the body’s stress response system and are interpreted as consistent with the Adaptive Calibration Model which suggests that cortisol regulation can have adaptive significance.
Keywords: bonding, parenting, cortisol, race, longitudinal
Introduction
Studies of the hypothalamic-pituitary-adrenal (HPA) axis have burgeoned over the past few decades, examining the adrenal end-product cortisol as an indicator of stress regulation. Cortisol is stress responsive, increasing after exposure to unpredictable, uncontrollable or social evaluative contexts (Dickerson & Kemeny, 2004). Cortisol’s release is dynamic even within a single day, such that high morning levels may help the individual prepare for the upcoming day, but high evening levels may contribute to impaired immune function and health problems (Adam, 2006; Shirtcliff et al., 2012; Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012). Across longer durations of time, the link between elevated cortisol and stress exposure does not necessarily persist, and instead low cortisol may be evinced in stressed individuals (Miller, Chen, & Zhou, 2007; Weems & Carrion, 2007; Yehuda et al., 1995). Less attention has been paid to how cortisol functions in social contexts that are warm, and involve sensitive and stable caregiving relationships (Adam, Klimes-Dougan, & Gunnar, 2007; Pendry & Adam, 2007; Tarullo & Gunnar, 2006), though recent theoretical models emphasize the powerful role of positive social interactions (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011) and postulate that attentive caregivers can protect children from the physiological impact of stressors (Shonkoff, 2010). Furthermore, a handful of studies illustrate these positive contexts can be enhanced through interventions to exert powerful influences on HPA development (Brotman et al., 2007; Cicchetti, Rogosch, Toth, & Sturge-Apple, 2011; Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008; Fisher, Stoolmiller, Gunnar, & Burraston, 2007; Luecken et al., 2010; van de Wiel, van Goozen, Matthys, Snoek, & van Engeland, 2004). Demonstrating an empirical link between positive parenting and HPA functioning is an important step towards illustrating whether positive social contexts can “get under the skin”. The present paper examines whether positive parenting experiences in adolescence prospectively predict cortisol functioning several years later during young adulthood.
The importance of the possible connection between positive family environments across phases of development and HPA regulation is underscored by Attachment Theory. Attachment theory postulates that qualities of early primary relationships influence later social-processing (Bowlby, 1988). Poor parent child connectedness or attachment is associated with a wide variety of problem behaviors (e.g., substance use, suicide attempts, depression, risky behavior) (Ackard, Neumark-Sztainer, Story, & Perry, 2006), whereas secure attachment often predicts positive outcomes (Boutelle, Eisenberg, Gregory, & Neumark-Sztainer, 2009). Secure attachment stems in part from early attentive and sensitive parenting (Thompson & Calkins, 1996). These primary social relationships provide the developing individual with the ability to form secure attachments to peers and partners later in life (Del Giudice, 2009). Likewise other theoretical models of social development emphasize that strong parent-child bonds during adolescence influence the child’s adoption of values, norms, and behaviors (Hawkins, Kosterman, Catalano, Hill, & Abbott, 2005). Parents who express positive emotions, reward positive behaviors, and make frequent attempts at calm positive communication foster greater bonding and secure attachment with their teens. Bonding to a positive parental figure, in turn, increases the likelihood that the adolescent will adopt pro-social values of their parents and avoid antisocial behaviors. Thus, parent-adolescent attachment may serve as a protective agent even in the context of stressful experiences and environments (Cleveland, Collins, Lanza, Greenberg, & Feinberg, 2010; Sousa et al., 2011; Stadler, Feifel, Rohrmann, Vermeiren, & Poustka, 2010).
One mechanism by which parental attachment and bonding influences later adjustment may be through their impact on the functioning of physiological stress responsive system (SRS). We believe positive caregiving environments ‘get under the skin’ in infancy. For instance, evidence indicates that secure attachment to a caregiver dampens the HPA response to stressors through social buffering. Stress is handled through a dyadic process between the caregiver and child, requiring less HPA response within the infant (Gunnar & Hostinar, 2015; Hostinar, Sullivan, & Gunnar, 2014). Furthermore, positive caregiving heightens other bonding-related physiological processes. For example, social relationships can strengthen systems that instantiate bonding (e.g., oxytocin release) which then serves to further dampen HPA responsivity (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Heinrichs & Gaab, 2007). Emotion- and stress-regulatory neurocircuitry (e.g., prefrontal cortex) is shaped by attachment relationships across development and may dampen HPA functioning during social interactions as the individual uses these bonds to cope with a stressor. This pattern is mirrored in studies on attachment quality and cortisol in infants and toddlers (Dozier, et al., 2008) which find elevated cortisol in insecurely attached children (Hertsgaard, Gunnar, Erickson, & Nachmias, 1995; Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996; Pendry & Adam, 2007). This may reflect more reliance on the infant’s own stress response system in the absence of support from a parent to whom the child is securely attached. This illustrates that during the first years of life and through multiple mechanisms, the SRS integrates environmental information to establish physiological set-points and calibrate physiological functioning (Adam, et al., 2007; Ellis, et al., 2011).
Unfortunately, less is known about how SRS are shaped by caregiving environments during adolescence or young adulthood (Doom, Hostinar, VanZomeren-Dohm, & Gunnar, 2015; Fagundes, Diamond, & Allen, 2012; Hostinar, Johnson, & Gunnar, 2015; Hostinar, et al., 2014). The story may be more complex by the time children develop into young adults (Quirin, Pruessner, & Kuhl, 2008) in part because a developmental task of the adolescent is to move away from the primary attachment figure and place more reliance on one’s own coping abilities(Steinberg, 2000), shifting from a dyadic regulatory process between caregiver and child toward more internal self-regulation (Hostinar & Gunnar, 2013). Indeed, some studies find low cortisol or blunted reactivity and rhythms if loss of attachment figure was experienced in conjunction with low care or parental desertion (Hagan, Luecken, Sandler, & Tein, 2010; Tyrka et al., 2008)10. These studies may seem counter to the social buffering model which predicts blunted cortisol reactivity in the context of high parent-child attachment in infancy.
One methodological challenge with the attachment theory framework is that attachment, bonding, caregiving and parenting are sometimes treated synonymously (Willinger, Diendorfer-Radner, Willnauer, Jorgl, & Hager, 2005) and the person-oriented differentiation of attachment styles suggests a unitary attachment type (Ainsworth, 1979; Jones, Cassidy, & Shaver, 2015). Within these categorical styles are hints toward a multidimensional construct that encompass a number of different attachment-related components with distinct behavioral underpinnings (e.g., secure base behaviors, environment exploration, proximity seeking) (Ainsworth, 1979). Measures of attachment often include continuous attachment indices. For example, the adult attachment interview considers whether the parent was loving, rejecting, neglectful, involved and/or pressuring (Jones, et al., 2015; Manassis, Owens, Adam, West, & Sheldon-Keller, 1999) and the parental bonding instrument includes continuous variables for care, protection and control behaviors (Parker, Tupling, & Brown, 1979). Nonetheless, because attachment is used as a unitary construct it is difficult to know what the important ingredients are – or whether all of them are important and add together to shape youth development. Therefore, the present study uses a multidimensional approach to measuring several positive aspects of the adolescent-parent relationship, including observational measures, which we hypothesize helps shape HPA functioning in young adulthood.
Our investigation is focused on two developmental stages – adolescence and emerging adulthood. Adolescence is a developmental switchpoint (Del Giudice, Ellis, & Shirtcliff, 2011; Ellis, Del Giudice, & Shirtcliff, 2012) initiated by pubertal maturation (Dahl, 2004) and other biological changes such as the reawakening of the HPA axis after a hypo-responsive period15,17. Collectively, these biological changes allow the adolescent to be more open to environmental change and potentially more sensitive to their context as the HPA recalibrates to new contextual challenges (Dahl & Gunnar, 2009) and for earlier environmental experiences to coalesce (Dahl, 2004; Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Shirtcliff & Ruttle, 2010). Adolescence terminates with the attainment of adult roles and responsibilities (Dahl, 2004). Young adults experience transitions away from their caregivers and towards independence (Steinberg, 2000), yet the biobehavioral impact of early experiences are expected to persist. Experiences within the family largely establish how children will navigate the transition to young adulthood, and this transition may be further influenced by proximate scaffolding from caregivers (Steinberg et al., 2006). Therefore, the quality and consistency of caregiving during adolescence is expected to influence set-points for stress physiology even after the transition to adulthood (Steinberg, 2000).
Building from our prior work (Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011b), the present study takes the vantage point that current stress regulation depends on the ‘background’ level of risk, threat or danger, and prior adaptation to context and salient events (Geronimus, Hicken, Keene, & Bound, 2006), including the culmination of prior adversity (Del Giudice, et al., 2011; Ellis, et al., 2012). Social conditions such as racial discrimination can heighten background threat and alter HPA functioning; therefore, race comparisons provide a window into the influence of background threat on the association between positive parenting and HPA regulation. As in previous studies where comparisons were made between teens in high vs. low risk environments (Cleveland, et al., 2010), we anticipate that the positive impact of parental attachment may not be as great in riskier environments. Typically, Blacks live in more threat-laden environments due to the persistence of interpersonal and structural discrimination. Risk can be magnified further if concomitant with lower access to resources, dangerous neighborhoods, less green space, substandard housing, fewer opportunities, substandard education, and other stressors which are more frequently experienced by Blacks (Mays, Cochran, & Barnes, 2007). Warm, responsive parenting may not be enough to overcome the greater exposure to background risk conveyed by discrimination and racial hassles. Within environments characterized by relative safety and in which parents are warm and positive, high cortisol may appear largely beneficial as it allows the individual to be maximally open to salient protective cues within relative safety (Shirtcliff, Peres, Dismukes, Lee, & Phan, 2014); our prior work found this “sensitive” profile was more often characteristic of Whites (Skinner, et al., 2011b) than Blacks. Our present investigation is not designed to explain race differences but to use race comparisons as a window into how contextual risk, danger and threat may influence the way in which positive parental attachment and bonding during adolescence are related to HPA functioning.
The present study examines whether positive parenting in adolescents predicts cortisol functioning six years later when youth become emerging adults. We examine four measures of positive parenting during adolescence: attachment, bonding, observed rewards for positive behavior, and teen perception of parent rewarding positive behavior. We analyze these measures as related yet potentially distinct indices which may have divergent or additive impacts on HPA functioning. We also test of potential race differences in these relationships within Black families and White families. Finally, we consider curvilinear associations between positive parenting and cortisol, consistent with theory (Del Giudice, et al., 2011; Ellis, et al., 2012) and our prior work (Skinner, et al., 2011b).
Methods
Participants
Participants were recruited from a longitudinal study which examined effectiveness of the Parents Who Care© prevention intervention for families (Haggerty, MacKenzie, Skinner, Harachi, & Catalano, 2006; Haggerty, Skinner, MacKenzie, & Catalano, 2007). The intervention’s goal was to teach parents how to engage children in family processes. It included parenting, youth, and family components in two different delivery formats. Parenting measures were captured pre-intervention; therefore, intervention effects are included as a control variable rather than a moderator or mediator. Parents of eighth-grade students in the Seattle, WA (USA) school district received a letter describing the study, and parents were contacted by phone. Families were informed that the study was testing a parenting program to see if it can help reduce teens behaviors, such as using drugs, alcohol, or violence, and that some parents would take part in the program and others would not. Participants were informed that they would be paid for their time to complete study activities ($15 individually for completing surveys, $50 for completing the video observations and up to $100 for completing the program). Eligibility included the parent identifying the teen as Black (or African American) or White (or European American), English as their primary language, and planned to live in the area for at least 6 months. Forty-six percent of families who received letters (N=331) consented (55% of Blacks and 40% of Whites) balanced by gender. Parents who refused were more likely to be White, married, and had a higher education. Questionnaires were self-administered using laptop computers equipped with Audio Computer Assisted Self Interview (ACASI) software. Parent-child dyads were videotaped while completing three structured interaction tasks. All procedures were approved by the University of Washington Institutional Review Board.
At follow-up (5 to 6 years after intervention), we attempted to contact all 331 young adults who then ranged in age from 19 to 22 (mean 19.7). Of the original participants, 301 (90.1%) completed the surveys (67 and 73 Black males and females, respectively; 82 and 79 White males and females, respectively). Two participants were not eligible for saliva collection due to incarceration. Of those surveyed 93.6% provided at least one useable saliva sample. Twelve samples were provided by 76.7% of those surveyed with no significant difference in compliance by race or gender. Most participants were enrolled in school (57.8%), while 45.6% were employed at least part time and 18% were currently neither employed nor attending school regularly.
Young Adult Cortisol Collection Procedure
Participants completed a 50-minute survey and were given instructions for collecting saliva and a collection kit (Fernades, Skinner, Woelfel, Carpenter, & Haggerty, 2012). Data collectors trained participants on research protocols to facilitate compliance. Type and timing of reminders were specific to each participant (Fernades, et al., 2012). At least 1.5 mL of saliva was collected by passive drool in each sample four times each day over 3 days: (1) when the participant woke up, before they were out of bed; (2) 30 minutes after the initial sample; (3) at least 1 hour after lunch; (4) at bedtime. Participants were asked to avoid eating, drinking, exercising, or brushing their teeth for 30 minutes before collection to avoid sample contamination. Participants completed diary entries immediately after each collection recording the date and time and answering questions evaluating demographic, social, emotional and physiological factors. Saliva samples were frozen until picked up by study staff, and then frozen at −80°C until assay. The participant was given $50 for completing the survey and received $5 per saliva sample (a maximum of $60 upon providing the samples).
Measures
Data on demographics and parenting were collected in the eighth grade. Parents reported on their child’s race on school enrollment forms. Cortisol was assayed on saliva collected at approximately age 20.
Cortisol
An enzyme-immunoassay was used to measure cortisol using 80μL of saliva. The assay uses a purified polyclonal anti-cortisol antibody, R4866, provided by C. Munro (U.C. Davis) and reference calibrators (Steraloids, cat. no. Q3880). This assay has been validated for use in saliva (Tomblingson, 2005). Intra- and inter-assay coefficients of variation were 2.4% and 13.3%, respectively. Results were further validated by comparison with a commercial kit (Assay Designs, cat. no.900-071), r(55) = 0.71, p<.0001. Cortisol was measured in micrograms per deciliter, with a lower detection limit of .03 μg/dL. Natural log transformation was used to reduce skew to a normal distribution.
Control Measures
Following Skinner and colleagues (Skinner, et al., 2011b), control variables included gender, family income during adolescence, and sleep duration. Income of the family at baseline (eighth grade) was used as an indicator of resources availability while growing up. This prospective measure of income may be a more stable indicator of lifetime socioeconomic status than concurrent income, which transiently and precipitously declines in emerging adults. Moreover, controlling for income partially rules out influence of stressor exposure stemming from earlier poverty or resource availability. Household per capita income was calculated from parent’s endorsement of 1 of 11 categories for annual household income (before taxes). We assigned the midpoint of the range and then divided by the number of people in the household as reported by parents (on average $7,807 for blacks, $21,970 for whites, race difference p<.01). The Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) as collected on each saliva day was used to determine sleep duration based on answers to “During the past week, how many hours of actual sleep did you get each night?” High scores indicate short sleep duration, and was greater on average in blacks (M=.94, SD=1.08) than whites (M=.82, SD=.97, p<.01).
Parent-teen attachment
Parent-teen attachment was measured using the sum of 28 items (alpha = .94) from teen-report on the Inventory of Parent and Peer Attachment (Armsden & Greenberg, 1987). Additional survey items were used to measure parent-teen bonding. The teen was asked 5 questions about their mother and father respectively (alpha=.87). When teens replied for two caregivers, the maximum of the two scores was used. Items included “Are you close to your mother?”, “Do you share your thoughts and feelings with your mother?”, “Do you get along with your mother?”, and “Do you want to be the kind of person your mother is?” Responses were provided on a 4 point scale, YES, yes, no, NO. High scores indicate higher attachment and bonding, on a continuous metric.
Parent rewarding positive behavior was measured in two ways
Teens were asked three questions (alpha .82): “Do your parents notice when you do well in school?”, “Do your parents notice when you help out at home?”, and “Do your parents notice when you do something well?” Responses were provided on the same 4-point scale where high scores indicate teens reported that parents noticed their good behavior. Rewards were also measured using ratings of observed behavior between parents and teens in videotaped interactions using the Social Development Model-Observational Coding System (Spagnolo et al., 2002). Prior to each videotaped interaction task, a research assistant set up the video equipment, provided oral and written standardized instructions to each family, and then left the room. Families completed three structured interaction tasks (1) a 10-minute warm-up interaction between one or both parents and the teen, (2) a 10-minute dyadic problem-solving interaction between a parent and the teen, and (3) a 5-minute dyadic recognition task during which the parent and teen told each other things they like about the other. Eighteen raters (5 men, 13 women; 28% African American, 66% European American, 6% Hispanic) completed an average of 93 hours of training. Ratings were made using 5-point Likert-type scales (Not at All, A Little, Sometimes, Often, Very Often). Inter-rater agreement across 20% of double-rated videos was high (ICC = .89) (Lindahl, 2001). Rewards for positive behavior was computed as the mean of seven items, including “Caregiver was warm and encouraging of teen’s opinions” and “Caregiver reinforced or rewarded teen’s prosocial behavior or attitudes.” Items were selected a priori and found to load on a single factor (alpha=.82).
Analyses
Hierarchical linear models (HLM) estimated the trajectory of cortisol across the day using SAS PROC MIXED (Singer, 1998). Repeated cortisol measures (max = 12/person) were nested within person. The two levels of the model were estimated using a random effect for intercept (to capture waking cortisol levels) and Time Since Waking (TSW, to capture the linear diurnal slope). The cortisol awakening response (CAR) was modeled using a fixed effect for a dummy variable coded 0/1 for samples collected between 25 and 55 min after waking. Cross-tabulation with tube number verified that 94.3% of these observations were the designated second tube of the day. Similarly, over 90% of the first tubes of the day were collected prior to 25 min after waking. Preliminary analyses tested for a within-person random effect of CAR, DAY and DAY × TSW and found no significant variation in these effects. The DAY × TSW effect was dropped and both DAY and CAR were treated as fixed effects, consistent with our earlier reports (Shirtcliff & Essex, 2008; Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011a). Intervention condition assignment was included as a control variable. Additional control variables (demographics, daily diary, medication, mood, etc) were examined to influence cortisol, but were not significant and were dropped from further analyses.
Race was entered as a main effect on waking cortisol (i.e. intercept, where TSW = 0), an interaction with CAR, and an interaction with TSW. Post hoc analyses included single degree of freedom significance tests of race differences in point estimates at waking, 30 min, 8 hr, and 16 hr after waking.
A two-level base model was estimated including intercept (i.e., waking cortisol levels), race, gender, CAR, TSW, income, and sleep duration, and the 2-way interactions of race with gender, CAR, and TSW. To this base model we added (1) a main effect for each of the four measures of positive parenting indicating the impact of each measure on the intercept (waking level); (2) the interaction of race with each predictor to determine whether the predictor was associated with waking levels differently for Whites than Blacks; (3) a predictor-squared term to assess possible non-linear associations between predictor and waking cortisol; (4) the interaction between race and predictor-squared to test race moderation in non-linear effects; and (5) the 3-way interactions (TSW × race × predictor) to assess whether each positive parenting predictor was associated with linear decline over the day differently for Whites and Blacks. Post hoc analyses were conducted separately by race.
Results
Descriptive Results
Race differences were evident for several demographic measures. Whites reported higher per capita income (Table 1) and parental education; Blacks reported higher prevalence of single parenthood. Most primary caregivers were female (>80%), with 71.6% being the adolescent’s biological mother. Caregiver relationships were similar across race with one exception: more Black youth had another female primary caregiver (e.g., grandmother, aunt) than did White youth (χ2(1)=13.95, p<.001). Analysis of Covariance (ANCOVA) results for race differences in level of the four positive parenting measures controlling for gender and family income are presented in Table 1. Only one difference was detected. Black teens reported significantly higher bonding to parents than did White teens (B=.13, p<.001). Bivariate correlations between parenting measures were significant. The three teen reports of parenting were moderately correlated (r=.64 attachment with bonding; r=.54 attachment with teen report of parent rewards; r=.47 bonding with teen report of rewards, ps<.0001) but each was correlated much more modestly with the observed measures (r=.13 with attachment; r=.08 with bonding; r=.13 with teen report of parent rewards, p<.0001). Results of race differences in diurnal cortisol have been previously published (Skinner, et al., 2011b). In summary cortisol was higher upon waking for Whites compared to Blacks (B=.09, p<.01). There were no gender effects or race by gender interactions predicting waking cortisol or the CAR. Cortisol’s diurnal decline was slower (flatter slope) for Blacks than Whites (B=.03, p<.001).
Table 1.
Descriptive statistics on demographics, predictors and control variables by race.
| Variable | Blacks | Whites | Total Sample |
|---|---|---|---|
| n=127 | n=147 | n=274 | |
| % female | 50% | 50% | 50% |
| 8th grade age | 13.66 (0.46) |
13.66 (0.37) |
13.66 (0.41) |
| Per capita income 8th grade | 8,218.00*** (10,131) |
22,411.00 (16,446) |
15,893.00 (15,587) |
| Sleep duration scale | 1.02* (1.13) |
0.85 (0.95) |
0.93 (1.03) |
| Attachment scale | 101.70 (19.49) |
104.42 (21.34) |
103.15 (20.50) |
| Bonding scale | 3.36*** (0.65) |
3.31 (0.59) |
3.33 (0.62) |
| Observed rewards | 2.99 (0.72) |
3.38 (0.69) |
3.20 (0.73) |
| Teen report rewards | 3.49 (0.69) |
3.47 (0.53) |
3.48 (0.61) |
Race difference significance (df=1) controlling for gender and income
p< .05;
p< .01;
p< .001.
Race moderation
Results for these analyses are summarized in Table 2. The model testing for race moderation included 16 interactions with race. Of these, 7 were statistically significant with p < .05 and 2 achieved trend level significance with p < .10. As previously reported, there was a significant race difference in linear decline in cortisol levels over the day such that the slope was flatter for Blacks compared to Whites. Income effects were also moderated by race in that higher income was associated with lower waking cortisol for Blacks but not Whites. Further race moderation was evidenced by statistically significant race interactions with the squared effect of attachment, the main effect for bonding, the main and squared effects for observed rewards, and the main and squared effects for teen report rewards. Based on these results, the models were estimated separately for Blacks and Whites. The results by race are reported in Table 3 and described in more detail below.
Table 2.
Estimated Effects of Parent-Teen Relationship Measures on Diurnal Cortisol during Emerging Adulthood
| Eighth-grade measure | Linear effect on waking level (Intercept) | Linear effect by race | Squared effect | Squared effect by race | On decline over the day (Slope) | On decline over the day (Slope) by race |
|---|---|---|---|---|---|---|
| P/T attachment | 0.00 | 0.00 | 0.00 | 0.0002+ | 0.00 | 0.00 |
| P/T bonding | 0.00 | 0.24* | 0.06 | −0.03 | −0.02+ | 0.00 |
| Observed rewards for positive behavior | −0.12** | 0.24*** | 0.00 | 0.02*** | 0.01 | −.01 |
| Teen report of rewards for positive behavior | 0.24** | −0.28* | 0.22*** | −0.26** | 0.00 | −0.02+ |
Note. P/T = Parent-teen.
p < .10
p < .05;
p < .01;
p < .001.
Table 3.
Estimated Effects of Parent-Teen Relationship Measures on Diurnal Cortisol during Emerging Adulthood separately for Blacks and Whites.
| Black | White | |||
|---|---|---|---|---|
| Effect | Estimate | p-value | Estimate | p-value |
| Intercept | −1.40 | <.0001 | −1.02 | <.0001 |
| Time Since Waking (TSW) | −0.04 | <.0001 | −0.08 | <.0001 |
| Cortisol Awaking Response (CAR) | 0.34 | <.0001 | 0.26 | <.0001 |
| Female | 0.04 | 0.59 | 0.07 | 0.28 |
| TSW × Female | 0.005 | 0.61 | 0.004 | 0.64 |
| CAR × Female | −0.18 | 0.10 | −0.05 | 0.55 |
| Income | −0.009 | 0.002 | −0.001 | 0.45 |
| Sleep Duration | 0.24 | 0.007 | 0.08 | 0.40 |
| Sleep Duration | 0.24 | 0.02 | 0.08 | 0.42 |
| Sleep Duration | 0.30 | 0.002 | 0.16 | 0.09 |
| Sleep Duration | 0.00 | 0.00 | ||
| Intervention Group – Control | 0.10 | 0.18 | −0.01 | 0.85 |
| Intervention Group – Self-administered | 0.25 | 0.0005 | 0.02 | 0.70 |
| Intervention Group – Group Administered | 0.00 | 0.00 | ||
| P/T attachment | −0.001 | 0.59 | −0.002 | 0.27 |
| P/T bonding | −0.03 | 0.72 | 0.23 | 0.0003 |
| Observed rewards | −0.11 | 0.008 | 0.13 | 0.001 |
| Teen report of rewards | 0.25 | 0.003 | −0.05 | 0.42 |
| P/T attachment squared | 0.00 | 0.66 | 0.0002 | 0.0001 |
| P/T bonding squared | 0.04 | 0.56 | 0.01 | 0.84 |
| Observed rewards squared | −0.005 | 0.18 | 0.01 | 0.0004 |
| Teen report of rewards squared | 0.25 | <.0001 | −0.07 | 0.41 |
| TSW × P/T attachment | 0.0002 | 0.44 | 0.0003 | 0.28 |
| TSW × P/T bonding | −0.02 | 0.06 | −0.02 | 0.05 |
| TSW × Observed rewards | 0.006 | 0.10 | −0.0002 | 0.91 |
| TSW × Teen report of rewards | 0.0005 | 0.95 | −0.02 | 0.02 |
Note: Shaded effects are those for which there is evidence of significant race moderation. Bolded effects are statistically significant.
Effects for Whites
We found that teen attachment was associated with waking cortisol levels, but this effect was non-linear. In models tested separately by race, the squared effect of teen reported attachment was significantly predictive of waking levels of cortisol (B=.0002, p < .0001) for Whites. Highly positive attachment reported by the teen was associated with higher waking cortisol 6 years later, and this effect was most apparent for individuals with very high attachment quality. Attachment was not related to the linear decline in cortisol over the day.
Higher bonding was associated with higher waking cortisol for Whites (B = .23, p = .0003). The effect of bonding on the diurnal slope was marginally significant and negative, indicating higher bonding was associated with steeper decline over the day (B = −.02, p = .09).
Greater observed rewards were associated with higher waking cortisol (B = .13, p = .001), especially at very high levels for Whites (B = .01, p = .0004). Effects on linear decline were not significant (B = −.0002, p = .91).
Teen reports of high rewards for positive behavior were not associated with waking levels of cortisol for Whites (B = −.06, p = .43; B = −.07, p = .41 for main and squared effects, respectively). Higher teen report of rewards were associated with steeper decline over the day for Whites (B = −.02, p = .02).
Effects for Blacks
No significant relationships were found for waking cortisol with teen report of attachment to parents (B = − .001, p = .59; B=.00003 p = .66 for main and squared effects, respectively) or bonding (B = −.02, p = .72; B=.04, p = .56 main and squared effects respectively). Attachment was not related to linear decline in cortisol over the day for Blacks (B = .0003, p = .45). The estimated effect of bonding on the diurnal slope was marginally significant and negative, indicating higher bonding was associated with steeper decline over the day (B = −.02, p = .09). For Blacks, there was a significant main effect for observed rewards for positive behavior, but in the negative direction (B = −.11, p = .008) indicating higher observed rewards were associated with lower waking cortisol. Effects on linear decline were not significant (B = .0006, p = .11).
Teen reports of high rewards for positive behavior were associated with higher waking cortisol (B = .25, p = .003) especially at the highest levels (B = .25, p = < .0001) for Blacks. Higher teen report of rewards were not associated with steeper decline over the day (B = .0005, p = .95).
Discussion
The relationship between positive teen-caregiver experiences during adolescence and physiological stress regulation during early adulthood is understudied despite the wide-held believe that positive parenting is important for youth development. We found that youth who had stronger positive parenting as adolescents had higher waking cortisol six years later than youth with lower positive parenting. HPA effects were especially pronounced for youth with the very highest amount of attachment, bonding or rewards. Effects were not overlapping such that the measures of positive parenting each had additive impact on HPA functioning even when simultaneously modeled; this suggests that measures of positive parenting are not all the same but rather may capture different or complimentary ways of building on attachment. Race moderated some of these effects. When examined separately by race, there were more hypothesized findings for whites than Blacks. This might seem unexpected, especially given that there were few mean differences in positive parenting measures between Blacks and Whites (Skinner, et al., 2011b). Below, we consider how findings about positive parenting effects on HPA functioning fit within an attachment framework or with recent theories about the adaptive calibration of stress responsivity.
Our finding that positive parenting measures additively predicted HPA functioning six years later fits with our expectation that parenting behaviors can “get under the skin” and that attachment is not a unitary construct but rather represents distinct, related behaviors that each incrementally impact HPA functioning. Our findings support this multidimensional approach through the use of both self-report and observed parenting measures. Our findings do not fit easily within a stress buffering framework (Heinrichs, et al., 2003). Instead, we found that higher cortisol was associated with positive parenting. This divergence can be explained in several ways. First, the attachment framework has largely been conceptualized with much younger children and there is a dearth of studies on adolescents and young adults, so it is possible that there is a reversal of HPA effects after the pubertal transition (Hostinar & Gunnar, 2013). Consistent with this is one study which found that parental responsivity was prospectively related to greater stress reactivity in adolescents (Hackman et al., 2013). Similarly, another study found that the provision of social support by a parent did not buffer HPA reactivity in adolescents but did within younger children (Hostinar, et al., 2015). Second, stress buffering may apply more closely to stress reactivity (Heinrichs, et al., 2003) rather than basal or morning levels in which elevated levels may be indicative of permissive effects (Sapolsky, Romero, & Munck, 2000). This suggests that the individual is prepared for their social environment, but is not actively mounting a stress response. Permissive effects implicate a different set of physiological actions largely mediated through the high affinity mineralocorticoid receptor (Joels, Sarabdjitsingh, & Karst, 2012; Zhou et al., 2010) rather than the low affinity glucocorticoid receptor that is responsible for terminating a stress response. Third, the attachment framework is, at its core, a developmental evolutionary model (Hrdy, 1999) which typically emphasizes tradeoffs and adaptations to context rather than favoring unidirectional or optimal outcomes (Shirtcliff, et al., 2014). Below, we consider how to interpret the study’s findings of high basal waking cortisol levels and positive parenting through such an evolutionary lens.
According to recent evolutionary models such as the Adaptive Calibration Model (ACM) (Boyce & Ellis, 2005; Del Giudice, et al., 2011), cortisol is not just a stress hormone which rises to help an individual cope with stressors or be vigilant against contextual signals of threat or danger. Cortisol is specifically attuned to social stressors, helping an individual encode and enhance salient signals, typically socially-charged settings. Many of these functions of cortisol are preparative or permissive and are long-lasting rather than acutely reactive to laboratory challenges. According to the ACM, it may be adaptive for an individual to express high waking cortisol (or steep diurnal rhythms) in low stress environments as high cortisol permits the individual to be sensitive to the positive and supportive elements of their environment. The costs of elevated cortisol are minimal as environmental forces such as an attentive caregiver or supportive social network would largely protect the individual from the impact of occasional minor stressors. High waking cortisol would benefit the individual by allowing them to maximally encode, learn and remember positive social and emotional cues, setting a sensitive individual up for optimal health and emotional wellbeing when they are in supportive contexts (Boyce & Ellis, 2005). Cortisol may help individuals to connect with one another (Taylor et al., 2000) across a range of social relationships (Gordon et al., 2008) and developmental stages (Del Giudice, 2009; Pierrehumbert et al., 2009). Recent empirical investigations are consistent with these beneficial effects of cortisol. High cortisol has been observed in contexts that are positive, social and enjoyable (Flinn, 2006); individuals with high cortisol levels or steep diurnal rhythms are especially empathic or socially-oriented (Adam & Gunnar, 2001; Booth, Granger, & Shirtcliff, 2008) and skilled at engaging in a reciprocal manner with other individuals (Buchanan, Bagley, Stansfield, & Preston, 2012) including several studies that have found that highly sensitive caregivers have elevated cortisol as their child accomplishes a difficult challenge, presumably protecting and supporting their child as they successfully navigated the task (Atkinson et al., 2013; Hibel, Granger, Blair, Finegood, & Family Life Project Key, 2015; Ruttle, Serbin, Stack, Schwartzman, & Shirtcliff, 2011; Sethre-Hofstad, Stansbury, & Rice, 2002). Thus, a sensitive or “open” HPA axis may be one in which the social or empathic connection between individuals in mild challenges or preparative basal states sets the stage for physiological setpoints. This sensitive profile, according to the ACM, would not be adaptive for individuals with greater life stress exposure.
Links between positive parenting during adolescence and high waking cortisol and steep slopes in young adulthood were largely limited to Whites and were typically null for Blacks. There were few differences between White and Black youth in the quality of parenting, attachment and bonding, so the null effects are not driven by race differences in quality of caregiving when youth were teens. Rather, according to the ACM, the advantages of an open biological sensitivity to context are limited to low stress, resource-rich contexts which unfortunately may be less common for Black youth (Skinner, et al., 2011b). Instead, a “buffered” profile may appear advantageous as lower cortisol levels allow the individual to encode only the most salient of stressors (Del Giudice, et al., 2011), avoiding constant assault by relatively unimportant irritations. This level of stress exposure may be characteristic of most Black youth (Mays, et al., 2007) who experience higher levels of environmental stressors than Whites as well as added strain of racism and racial discrimination. Our prior study (Skinner, et al., 2011b) and parallel work (Theall, Drury, & Shirtcliff, in press) found that Blacks were exposed to significantly more stressors than Whites in nearly every category of stressor. This is not meant to imply that Black youth in our study were buffered from positive parenting (which they received as much or more of than Whites on average); that type of interpretation for an “optimal” HPA profile is not consistent with evolutionary models. Instead, we contend that a supportive family environment in a moderately stressful context might promote an adaptation to a difficult world. In this case, higher quality parent attachment would be expected to be linked with a “closed” HPA axis, with parenting styles supporting a necessary buffering to contextual challenges.
The study is limited in relying on race to index background risk and understand health disparities (although we also controlled for income to minimize effect of socioeconomic status). More nuanced composites of adversity may be advantageous, yet also appear limited in that they have not fully explained race differences in extant literature (Cohen et al., 2006; DeSantis et al., 2007). Another limitation is that we are unable to determine why positive parenting was associated with HPA functioning six years later. It is difficult to know if results are due to parenting before, during, or after adolescence; controlling for the intervention somewhat addressed this concern, though not physiologically. Obtaining cortisol information at prior developmental stages could have helped elucidate these pathways. Regardless, it is important to show long-lasting effects into emerging adulthood.
Beyond the background context of being White or Black, the social context may influence HPA functioning in predictable ways, helping the individual’s physiology to encode the most salient aspects of their environment. In low risk environments, this may entail a hormone profile in which high cortisol helps the individual remain sensitive to all the positive, warm, and nurturing aspects of their caregiving experiences; these beneficial effects appear to extend across adolescence and into young adulthood when the caregiving environment may seem less important. The findings have important bearing on our understanding of the potential for positive parenting to impact cortisol functioning years later.
Figure 1.
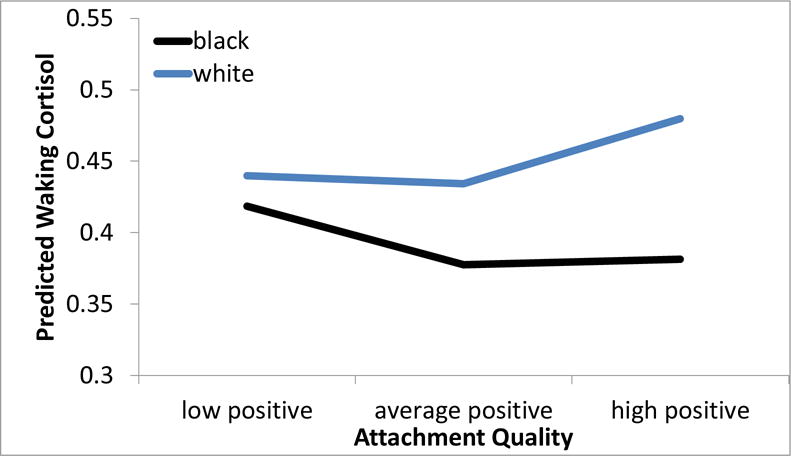
Teen-reported parent-child attachment quality was associated with higher waking cortisol levels six years later, but only within White young adults, and the effect was most pronounced within youth with the highest positive social environments as adolescents. Predicted Empirical Bayes scores are shown for one standard deviation above and below the mean attachment quality.
Figure 2.
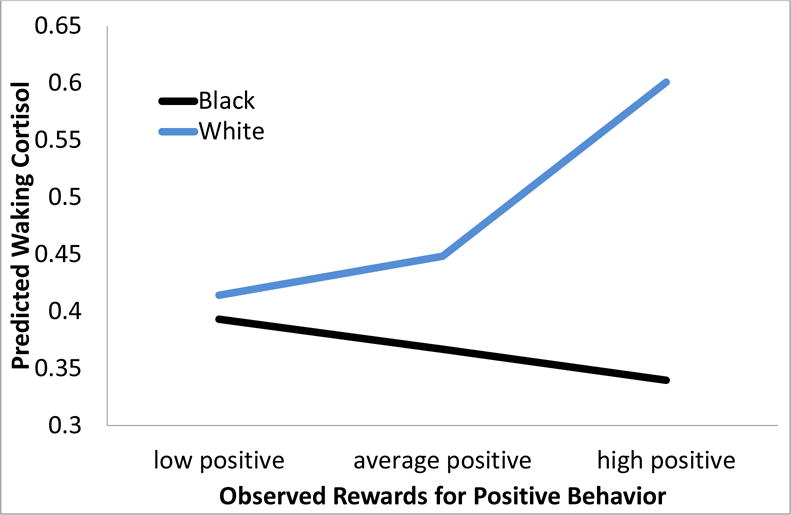
Observed Rewards for Positive Behavior (when youth were 8th graders) predicted higher waking cortisol levels six years later within White Young Adults, especially those with the highest rewards, but lower waking cortisol within Black Young Adults.
Figure 3.
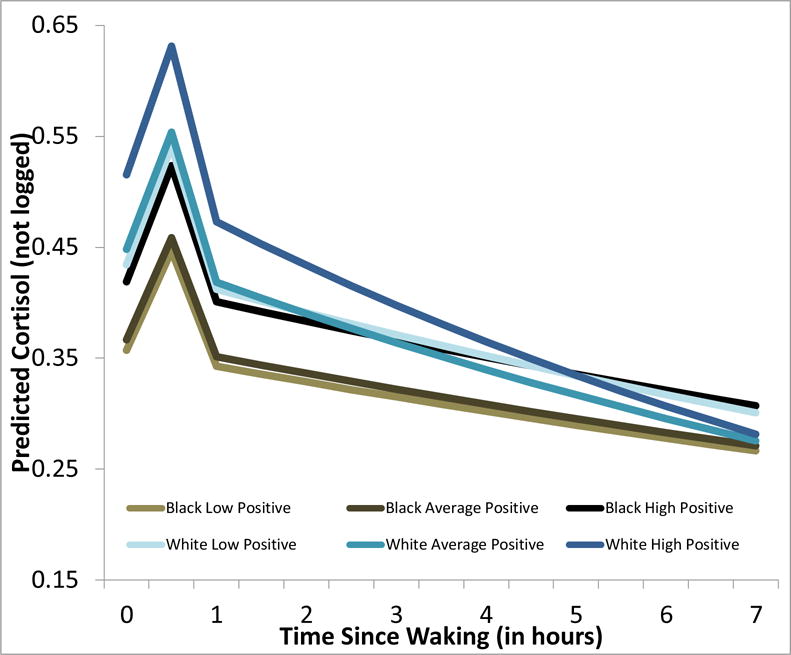
Teen-Report of how often their Parent Rewards Positive Behavior are associated with higher waking cortisol levels six years later, especially those with the most positive environments. Furthermore, Teen-Report of Reward was associated with steeper cortisol diurnal rhythm, but only within White Young Adults.
Research Highlights.
Positive Parenting Predicts cortisol functioning in young adults
When the link between positive parenting and cortisol was moderated by race, the effect was more consistent for White than Black youth.
Several parenting measures, including observational measures, were related independently to cortisol functioning, suggesting an additive benefit of multiple forms of bonding, attachment and parental rewards.
Acknowledgments
This study was supported by the National Institute of Drug Abuse (R01 DA021737, Haggerty, PI). Salary support for EAS was provided by National Institute of Mental Health (K01 MH093675) and for EMO by the National Institute of Drug Abuse (P30 DA027827).
Contributor Information
Elizabeth A. Shirtcliff, Human Development Family Studies, Iowa State University.
Martie L. Skinner, Social Development Research Group, University of Washington-Seattle.
Ezemenari M. Obasi, University of Houston, Department of Educational Psychology.
Kevin P. Haggerty, Social Development Research Group, University of Washington-Seattle.
References
- Ackard DM, Neumark-Sztainer D, Story M, Perry C. Parent-Child Connectedness and Behavioral and Emotional Health Among Adolescents. American Journal of Preventive Medicine. 2006;30(1):59–66. doi: 10.1016/j.amepre.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar M. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26(2):189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Klimes-Dougan B, Gunnar M. Social regulation of the adrenocortical response to stress in infants, children and adolescents. In: Coch D, Dawson G, Fischer KW, editors. Human behavior and the developing brain: Atypical development. New York, NY: Guilford Press; 2007. pp. 264–304. [Google Scholar]
- Ainsworth MD. Infant–mother attachment. Am Psychol. 1979;34(10):932–937. doi: 10.1037//0003-066x.34.10.932. [DOI] [PubMed] [Google Scholar]
- Armsden GC, Greenberg MT. The Inventory of Parent and Peer Attachment: Individual differences and their relationship to psychological well-being in adolescence. [Empirical Study] Journal of Youth & Adolescence. 1987;16(5):427–454. doi: 10.1007/BF02202939. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Gonzalez A, Kashy DA, Santo Basile V, Masellis M, Pereira J, Levitan R. Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology. 2013;38(12):2943–2951. doi: 10.1016/j.psyneuen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Booth A, Granger DA, Shirtcliff EA. Gender- and age-related differences in the association between social relationship quality and trait levels of salivary cortisol. Journal of Research on Adolescence. 2008;18(2):239–260. [Google Scholar]
- Boutelle K, Eisenberg ME, Gregory ML, Neumark-Sztainer D. The reciprocal relationship between parent-child connectedness and adolescent emotional functioning over 5 years. Journal of Psychosomatic Research. 2009;66(4):309–316. doi: 10.1016/j.jpsychores.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Bowlby J. A secure base: Parent-child attachment and healthy human development. London: Routledge; 1988. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Arch Gen Psychiatry. 2007;64(10):1172–1179. doi: 10.1001/archpsyc.64.10.1172. 64/10/1172 [pii] [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Bagley SL, Stansfield RB, Preston SD. The empathic, physiological resonance of stress. Soc Neurosci. 2012;7(2):191–201. doi: 10.1080/17470919.2011.588723. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, 3rd, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. doi: 0165-1781(89)90047-4 [pii] [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Dev Psychopathol. 2011;23(3):789–800. doi: 10.1017/S0954579411000307. S0954579411000307 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland MJ, Collins LM, Lanza ST, Greenberg MT, Feinberg ME. Does Individual Risk Moderate the Effect of Contextual-Level Protective Factors? A Latent Class Analysis of Substance Use. Journal of Prevention & Intervention in the Community. 2010;38(3):213–228. doi: 10.1080/10852352.2010.486299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. 68/1/41 [pii] [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21(1):1–6. doi: 10.1017/S0954579409000017. S0954579409000017 [pii] [DOI] [PubMed] [Google Scholar]
- Del Giudice M. Sex, attachment, and the development of reproductive strategies. Behav Brain Sci. 2009;32(1):1–21. doi: 10.1017/S0140525X09000016. discussion 21–67. doi: S0140525X09000016 [pii] [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. S0149-7634(10)00196-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. S1054-139X(07)00137-1 [pii] [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohm AA, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology. 2015;59:102–111. doi: 10.1016/j.psyneuen.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau JP, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Dev Psychopathol. 2008;20(3):845–859. doi: 10.1017/S0954579408000400. S0954579408000400 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Dev Psychopathol. 2011;23(1):7–28. doi: 10.1017/S0954579410000611. S0954579410000611 [pii] [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Shirtcliff E. Beyond allostatic load: The stress response system as a mechanism of conditional adaptation. In: Beauchaine TP, Hinshaw SP, editors. Child and Adolescent Psychopathology. 2nd. New York: Wiley & Sons; 2012. [Google Scholar]
- Fagundes CP, Diamond LM, Allen KP. Adolescent attachment insecurity and parasympathetic functioning predict future loss adjustment. Pers Soc Psychol Bull. 2012;38(6):821–832. doi: 10.1177/0146167212437429. 0146167212437429 [pii] [DOI] [PubMed] [Google Scholar]
- Fernades A, Skinner ML, Woelfel T, Carpenter T, Haggerty K. Implementing self-collecting of biological specimens with a diverse sample. Field Methods. 2012;25(1):58–73. doi: 10.1177/1525822X12453526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905. doi: 10.1016/j.psyneuen.2007.06.008. S0306-4530(07)00143-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn MV. Evolution and ontogeny of stress response to social challenges in the human child. Developmental Review. 2006;26:138–174. [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health. 2006;96(5):826–833. doi: 10.2105/ajph.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45(3):349–352. doi: 10.1111/j.1469-8986.2008.00649.x. SYP649 [pii] [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE. The Social Buffering of the Hypothalamic-Pituitary-Adrenocortical Axis in Humans: Developmental and Experiential Determinants. Soc Neurosci. 2015 doi: 10.1080/17470919.2015.1070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. S0954579409000054 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Kobrin L, Hurt H, Farah MJ. Selective impact of early parental responsivity on adolescent stress reactivity. PLoS One. 2013;8(3):e58250. doi: 10.1371/journal.pone.0058250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MJ, Luecken LJ, Sandler IN, Tein JY. Prospective effects of post-bereavement negative events on cortisol activity in parentally bereaved youth. Dev Psychobiol. 2010;52(4):394–400. doi: 10.1002/dev.20433. [DOI] [PubMed] [Google Scholar]
- Haggerty KP, MacKenzie EP, Skinner ML, Harachi TW, Catalano RF. Participation in “parents who care”: predicting program initiation and exposure in two different program formats. J Prim Prev. 2006;27(1):47–65. doi: 10.1007/s10935-005-0019-3. [DOI] [PubMed] [Google Scholar]
- Haggerty KP, Skinner ML, MacKenzie EP, Catalano RF. A randomized trial of Parents Who Care: Effects on key outcomes at 24-month follow-up. Prevention Science. 2007;8(4):249–260. doi: 10.1007/s11121-007-0077-2. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Kosterman R, Catalano RF, Hill KG, Abbott RD. Promoting positive adult functioning through social development intervention in childhood: Long-term effects from the Seattle Social Development Project. Archives of Pediatrics and Adolescent Medicine. 2005;159(1):25–31. doi: 10.1001/archpedi.159.1.25. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. doi: S0006322303004657 [pii] [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr Opin Psychiatry. 2007;20(2):158–162. doi: 10.1097/YCO.0b013e3280146a13. 00001504-200703000-00012 [pii] [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Dev. 1995;66(4):1100–1106. [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Finegood ED, Family Life Project Key, I Maternal-child adrenocortical attunement in early childhood: Continuity and change. Dev Psychobiol. 2015;57(1):83–95. doi: 10.1002/dev.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. Future directions in the study of social relationships as regulators of the HPA axis across development. J Clin Child Adolesc Psychol. 2013;42(4):564–575. doi: 10.1080/15374416.2013.804387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev Sci. 2015;18(2):281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy SB. Mother nature: Maternal instincts and how they shape the human species. New York: Ballantine books; 1999. [Google Scholar]
- Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64(4):901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- Jones JD, Cassidy J, Shaver PR. Parents’ self-reported attachment styles: a review of links with parenting behaviors, emotions, and cognitions. Pers Soc Psychol Rev. 2015;19(1):44–76. doi: 10.1177/1088868314541858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl KM. Methodological issues in family observational research. In: Kerig PK, Lindahl KM, editors. Family observational coding systems: Resources for systemic research. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 23–32. [Google Scholar]
- Luecken LJ, Hagan MJ, Sandler IN, Tein JY, Ayers TS, Wolchik SA. Cortisol levels six-years after participation in the Family Bereavement Program. Psychoneuroendocrinology. 2010;35(5):785–789. doi: 10.1016/j.psyneuen.2009.11.002. S0306-4530(09)00327-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassis K, Owens M, Adam KS, West M, Sheldon-Keller AE. Assessing attachment: convergent validity of the adult attachment interview and the parental bonding instrument. Aust N Z J Psychiatry. 1999;33(4):559–567. doi: 10.1080/j.1440-1614.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu Rev Psychol. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. 2006-23058-002 [pii] [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A Parental Bonding Instrument. British Journal of Medical Psychology. 1979;52(1):1–10. doi: 10.1111/j.2044-8341.1979.tb02487.x. [DOI] [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. International Journal of Behavioral Development. 2007;31(3):218–231. doi: 10.1177/0165025407074634. [DOI] [Google Scholar]
- Pierrehumbert B, Torrisi R, Glatz N, Dimitrova N, Heinrichs M, Halfon O. The influence of attachment on perceived stress and cortisol response to acute stress in women sexually abused in childhood or adolescence. Psychoneuroendocrinology. 2009;34(6):924–938. doi: 10.1016/j.psyneuen.2009.01.006. S0306-4530(09)00026-2 [pii] [DOI] [PubMed] [Google Scholar]
- Quirin M, Pruessner JC, Kuhl J. HPA system regulation and adult attachment anxiety: individual differences in reactive and awakening cortisol. Psychoneuroendocrinology. 2008;33(5):581–590. doi: 10.1016/j.psyneuen.2008.01.013. S0306-4530(08)00037-1 [pii] [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Serbin LA, Stack DM, Schwartzman AE, Shirtcliff EA. Adrenocortical attunement in mother-child dyads: Importance of situational and behavioral characteristics. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.06.014. S0301-0511(11)00177-3 [pii] [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27(6):731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2012;54(5):493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Development and Psychopathology. 2008;50(7):691–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, Phan JM. Riding the physiological roller coaster: Adaptive significance of cortisol stress reactivity to social contexts. Journal of Personality Disorders. 2014;28(1):65–76. doi: 10.1521/pedi.2014.28.1.40. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Ruttle P. Immunological and neuroendocrine dysregulation following early deprivation and stress. In: Brisch KH, editor. Attachment and Early Disorders of Development. Munich: Klett-Cotta, Stuttgart; 2010. [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. CDEV1399 [pii] [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23(4):323–355. [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology. 2011a;23(4):1167–1186. doi: 10.1017/S095457941100054X. S095457941100054X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011b;23(4):1167–1186. doi: 10.1017/S095457941100054X. S095457941100054X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa C, Herrenkohl TI, Moylan CA, Tajima EA, Klika JB, Herrenkohl RC, Russo MJ. Longitudinal study on the effects of child abuse and children’s exposure to domestic violence, parent-child attachments, and antisocial behavior in adolescence. Journal of Interpersonal Violence. 2011;26(1):111–136. doi: 10.1177/0886260510362883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo B, DeLoach CM, Haggerty KP, Hill KG, Weaver-Randall K, Catalano RF, Hawkins JD. The Social Development Model Observational Coding System. Seattle: University of Washington, Social Development Research Group; 2002. [Google Scholar]
- Stadler C, Feifel J, Rohrmann S, Vermeiren R, Poustka F. Peer-Victimization and Mental Health Problems in Adolescents: Are Parental and School Support Protective? Child Psychiatry & Human Development. 2010;41(4):371–386. doi: 10.1007/s10578-010-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Gallagher lecture. The family at adolescence: transition and transformation. J Adolesc Health. 2000;27(3):170–178. doi: 10.1016/s1054-139x(99)00115-9. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine D. The study of developmental psychopathology in adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Handbook of developmental psychopathology. 2nd. Vol. 2. Hoboken, NJ: Wiley; 2006. pp. 710–741. [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral response to stress in females: Tend and befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Theall K, Drury S, Shirtcliff EA. Cumulative neighborhood risk and allostatic load in adolescents. doi: 10.1093/aje/kws185. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA, Calkins SD. The double-edged sword: Emotional regulation for children at risk. Development and Psychopathology. 1996;8(01):163–182. doi: 10.1017/S0954579400007021. [DOI] [Google Scholar]
- Tomblingson G. Comparison of paired urinary and salivary cortisol measures as biomarkers of stress; Paper presented at the University of Washington Undergraduate Research Symposium; Seattle, WA. 2005. [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63(12):1147–1154. doi: 10.1016/j.biopsych.2008.01.011. S0006-3223(08)00105-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wiel NM, van Goozen SH, Matthys W, Snoek H, van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2004;43(8):1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Horm Behav. 2012;62(1):36–42. doi: 10.1016/j.yhbeh.2012.04.014. S0018-506X(12)00136-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, Carrion VG. The association between PTSD symptoms and salivary cortisol in youth: the role of time since the trauma. J Trauma Stress. 2007;20(5):903–907. doi: 10.1002/jts.20251. [DOI] [PubMed] [Google Scholar]
- Willinger U, Diendorfer-Radner G, Willnauer R, Jorgl G, Hager V. Parenting stress and parental bonding. Behav Med. 2005;31(2):63–69. doi: 10.3200/BMED.31.2.63-72. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Zhou M, Bakker EH, Velzing EH, Berger S, Oitzl M, Joels M, Krugers HJ. Both mineralocorticoid and glucocorticoid receptors regulate emotional memory in mice. Neurobiol Learn Mem. 2010;94(4):530–537. doi: 10.1016/j.nlm.2010.09.005. [DOI] [PubMed] [Google Scholar]


