Abstract
Novel interventions that reestablish endogenous insulin secretion and thereby halt progressive end‐organ damage and prolong survival of patients with autoimmune Type 1 diabetes mellitus (T1DM) are urgently needed. While this is currently accomplished with allogeneic pancreas or islet transplants, their utility is significantly limited by both the scarcity of organ donors and life‐long need for often‐toxic antirejection drugs. Coadministering islets with bone marrow‐derived mesenchymal stem cells (MSCs) that exert robust immune‐modulating, anti‐inflammatory, anti‐apoptotic, and angiogenic actions, improves intrahepatic islet survival and function. Encapsulation of insulin‐producing cells to prevent immune destruction has shown both promise and failures. Recently, stem cell‐derived insulin secreting β‐like cells induced euglycemia in diabetic animals, although their clinical use would still require encapsulation or anti‐rejection drugs. Instead of focusing on further improvements in islet transplantation, we demonstrate here that the intraperitoneal administration of islet‐sized “Neo‐Islets” (NIs), generated by in vitro coaggregation of allogeneic, culture‐expanded islet cells with high numbers of immuno‐protective and cyto‐protective MSCs, resulted in their omental engraftment in immune‐competent, spontaneously diabetic nonobese diabetic (NOD) mice. This achieved long‐term glycemic control without immunosuppression and without hypoglycemia. In preparation for an Food and Drug Administration‐approved clinical trial in dogs with T1DM, we show that treatment of streptozotocin‐diabetic NOD/severe combined immunodeficiency mice with identically formed canine NIs produced durable euglycemia, exclusively mediated by dog‐specific insulin. We conclude that this novel technology has significant translational relevance for canine and potentially clinical T1DM as it effectively addresses both the organ donor scarcity (>80 therapeutic NI doses/donor pancreas can be generated) and completely eliminates the need for immunosuppression. Stem Cells Translational Medicine 2017;6:1631–1643
Keywords: Control of type 1 diabetes, Mesenchymal stem cells, Neo‐Islets, Three‐dimensional cell aggregates, Intraperitoneal transplantation, Immune isolation of allogeneic islet cells
Significance Statement.
Mesenchymal stem cells (MSCs) possess potent immune‐modulating, anti‐inflammatory, pro‐survival, and repair‐stimulating activities. In patients with juvenile Type 1 diabetes mellitus (T1DM), the insulin‐producing cells of the pancreas are destroyed by auto‐immune attacks. Treatment with insulin, a pancreas or pancreatic islet transplant will enhance patient survival and reduce serious complications. However, transplants depend on potentially toxic anti‐rejection drugs, and there is a shortage of pancreas donors. We tested whether the therapeutic activities of MSCs could be harnessed by combining them with healthy islet cells in cell clusters (“Neo‐Islets”) that are administered to a mouse model of T1DM. We show that Neo‐Islets eliminate the need for exogenous insulin in experimental T1DM and conclude that this novel therapy has significant promise for the treatment of veterinary and human T1DM.
Introduction
The clinical need for novel technologies that effectively treat patients with Type 1 diabetes mellitus (T1DM) and render them insulin‐independent is great and well documented. Endogenous insulin replacement by pancreas or islet of Langerhans transplants is currently the only treatment that can achieve insulin‐independence and provide significant end organ protection in patients with autoimmune‐mediated T1DM. However, the great shortage of suitable pancreas donors combined with the need for repeated islet transplants, requiring up to five donors each, continue to limit the general availability of these expensive therapies 1, 2. In addition, both transplant modalities depend on the permanent use of potentially toxic antirejection drugs 3, 4, 5, 6.
Novel approaches to address these major limitations of islet transplantation therapies have shown significant progress. Auto‐ and allo‐immune isolation of transplanted islet cells (ICs) is currently tested with various encapsulation technologies. Several of these are showing promise while others have failed due to foreign body reactions 7, 8, 9, 10, 11. When insulin‐producing β‐cells are culture expanded through outgrowth from freshly isolated islets, they progressively de‐differentiate and lose their ability to secrete insulin 12, 13, 14. Although partial in vitro redifferentiation is feasible, this process is relatively inefficient 15. For this reason, pancreatic progenitor, embryonic stem and induced pluripotent stem cell lines have recently been successfully used to generate cells that closely resemble β‐cells and that induce euglycemia in diabetic animal models 16, 17, while their therapeutic use would still require either encapsulation or anti‐rejection drugs. Other significant preclinical studies used the pleiotropic actions of bone marrow or adipose‐derived mesenchymal stem cells (MSCs), that is, their well‐documented immune‐modulating, anti‐inflammatory and complex trophic activities, and showed that the survival and function of transplanted islets was improved when islets were cocultured with MSCs and coadministered with islets or when administered islets were precoated with MSCs 18, 19, 20, 21, 22. A clinical trial in which MSCs alone were administered to patients with T1DM demonstrated a modest improvement in β‐cell function, a response that was previously observed in preclinical studies 23. This approach by several groups clearly demonstrated that inclusion of MSCs in islet transplantation technologies does modestly reduce the number of needed islet donors.
Mindful of these important observations, we chose in the current study a new strategy that did not focus on the further improvement of islet transplantation technologies but instead tested whether the inclusion of higher numbers of healthy MSCs (adipose or bone marrow derived) in freshly formed “Neo‐Islets” (NIs), Three‐dimensional (3D) aggregates of culture‐expanded allogeneic ICs and MSCs, could be used to potentiate the pleiotropic effects that the small numbers of MSCs, as pericytes, physiologically exert in islets 24. In this fashion, we reasoned a substantially expanded MSC component (from ∼2% in normal isles to ∼50%) in these NIs should immune‐protect, through close range signaling, culture‐expanded and coaggregated islet and stem cells in vivo. In addition, we postulated that this approach would also make available the robust anti‐apoptotic paracrine actions of MSCs and their released nanovesicles to neighboring ICs, combined with their pro‐angiogenic and potent anti‐inflammatory activities 25. Together, allogeneic NIs that are engineered in this fashion should possess, we hypothesized, the ability to provide adequate auto‐ and allo‐immune isolation of their cell components in vivo by creating a protective microenvironment where culture expanded ICs can resume their physiological endocrine and other functions, that is, reestablish euglycemia in the clinically relevant NOD mouse model of auto‐immune T1DM.
Accordingly, NIs of approximate islet size were generated in vitro from culture expanded, dedifferentiated ICs 12, 13, 14 and bone marrow‐derived MSCs of C57Bl/6 mice. NIs were administered to spontaneously diabetic, immune‐competent NOD mice that develop auto‐immune T1DM that largely resembles human T1DM 26. This allogeneic treatment protocol was chosen as it models the most common clinical situation in recipients of pancreas or islet transplants. By not using anti‐rejection drugs or encapsulation devices, we directly tested our hypothesis that high numbers of MSCs in NIs do enable ICs to survive and redifferentiate into normally functioning endocrine cells. This treatment established long‐term glycemic control in NOD mice, which demonstrates that NIs survive, engraft and redifferentiate into functional endocrine cells in vivo, and that both allo‐ and auto‐immune protection is achieved. Importantly, following i.p. administration the NIs were taken up by the well‐vascularized omentum 27, 28 where they engrafted long term and redifferentiated into physiologically insulin‐secreting cells, delivering insulin into the portal system of the liver 29. Simultaneously, re‐expression of other islet‐specific hormones occurred. Identical injection of NIs into nondiabetic animals resulted in omental engraftment without causing hypoglycemia, further demonstrating regulated islet hormone secretion. In preparation for a pilot study in pet dogs with T1DM (INAD # 021776), streptozotocin‐diabetic nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were treated by identical protocol with canine NIs (cNIs). In these, euglycemia was readily and durably induced and intraperitoneal Glucose Tolerance Tests (i.p. GTTs) were normalized by the exclusive release of canine‐specific insulin. Taken together, the present data demonstrate that the complex pleiotropic actions of MSCs, as hypothesized, can be readily harnessed to protect cultured ICs, and when coaggregated with them in NIs and administered i.p., facilitate long‐term glycemic control in mice with autoimmune T1DM. We conclude that these novel observations have significant translational relevance for the treatment of both canine and human T1DM.
Materials and Methods
Reagents
All reagents used and their sources are listed in Supporting Information Table S1.
Cell Isolation and Culture
Islets and adipose derived MSCs were Isolated and cultured from mice and dogs as previously reported 30, 31, 32, 33, 34. Purified human islets from nondiabetic subjects (Prodo Laboratories, Irvine, CA), and human adipose derived MSCs (P1, Lonza, Walkersville, MD) were identically cultured. See Supporting Information data. Prior to NI formation, cultured MSCs were characterized as in our previous publication 35. Cell viability was assessed using fluorescein diacetate (FDA) and propidium iodide as per the manufacturers' instructions.
Induction of Indoleamine 2,3 Dioxygenase
Canine MSCs were tested by rtPCR at passage 2 (P2) for induction of indoleamine 2,3 dioxygenase (IDO‐1) in response to overnight culture in DMEM‐F12 (Sigma, www.sigmaaldrich.com) +10% canine serum (Golden West Biologicals, www.goldenwestbio.com) + 10 ng/ml canine interferon gamma (IFNγ, R&D Systems, www.rndsystems.com). Results from IFNγ treated cultures were normalized to those of identically passaged and cultured (but without IFNγ) cells, and expressed as Log10RQ (n = 4 independent experiments).
Neo‐Islet Formation
MSCs (P1 to P5) and ICs (P1 to P2) were cocultured in DMEM‐F12 + 10% Fetal Bovine Serum (FBS; Hyclone, www.fishersci.com, for murine or human cells) or dog serum (for canine cells) at a 1:1 ratio in ultra‐low adhesion surface culture dishes (Corning, www.corning.com), and NIs formed overnight. Control MSC and Islet cell clusters were formed by the same method. Prior to in vivo administration, NIs were tested by reverse transcription polymerase chain reaction (rtPCR) for expression of islet and MSC associated genes (see below), and Fluorescence‐activated cell sorting (FACS) for determining the ratio of MSCs to ICs post‐formation.
Staining of Cells and Neo Islets
Where indicated, MSCs were stained with Cell Tracker Green (green, Life Technologies, www.thermofisher.com), and passaged ICs were stained with Lipophilic Tracer DiI (red, Life Technologies) according to the manufacturers' instructions.
Neo‐Islet Cellular Ratio Assessment
NIs were formed overnight from cell tracker green stained MSCs and unstained ICs, collected and dissociated to single cell preparations (30 minutes Accumax Innovative Cell Technologies, www.accutase.com), and analyzed by FACS (BD FACScan Analyzer, BD Biosciences, www.bdbiosciences.com) for percent green (MSCs) versus unstained (ICs) cells.
Immunohistochemistry
Harvested organs were fixed, paraffin embedded, sectioned and deparaffinized as previously described 35, then stained for indicated antigens by standard methods (see Supporting Information data).
rtPCR
RNA was extracted from 1x10e6 cells (Qiagen RNeasy Mini Kit, Qiagen, www.qiagen.com). Reverse transcription was performed using SuperScript II Reverse Transcriptase (Applied Biosystems, www.thermofisher.com) for 60 minutes at 42°C. rtPCR was carried out in duplicate using species‐specific TaqMan primers (Applied Biosystems; see Supporting Information Table S2) and the ABS 7500 Real Time PCR System. RQ was calculated through normalization to internal controls (beta actin and beta 2 microglobulin, and the machine's software. Results are presented as log10(RQ) ± log10(RQmin and RQmax). Differences greater or less than log10(RQ) 2 or −2 were considered significant. (see Supporting Information data).
Animal Care and Models
Animal studies were conducted in adherence to the NIH Guide for the Care and Use of Laboratory Animals, and were supervised and approved by an institutional veterinarian and member of the IACUC. Mouse experiments used female (a) C57Bl/6 (Harlan, www.envigo.com), (b) NOD (Jackson Laboratory, www.jax.org), (c) Nonobese diabetic/severe combined immunodeficiency mice (NOD/SCID, Harlan), weighing between 15 and 35 g. All treatments were conducted under isoflurane anesthesia. Care and anesthesia details are in Supporting Information .
Insulin Treatment
Where indicated, insulin was administered via slow‐release, subcutaneous insulin pellets (Linbits, LinShin, www.linshincanada.com; see Supporting Information data) following the manufacturer's instructions.
Blood Glucose Monitoring
In all in vivo studies, blood glucose concentrations were assessed twice per week via tail vein sampling, using a OneTouch Ultra 2 glucometer (Johnson and Johnson, www.jnj.com, level of detection, 20–600 mg glucose/dl).
Spontaneous Diabetes
Female NOD mice develop T1DM spontaneously between 12 and 20 weeks of age. Diabetes was confirmed by nonfasting blood glucose levels of >300 mg/dl on 3 separate days. Mice entered experimental or control groups at ages 13–21 weeks of age.
Streptozotocin‐Induced T1DM
C57Bl/6 and NOD/SCID mice were rendered diabetic with 3–5 i.p. doses (1 per day) of 50–75 mg/kg body weight (b.wt.) STZ (Sigma), freshly dissolved in 20 mM citrate buffer, pH 4.5, and randomized after diabetes was confirmed as above.
Treatment Protocols
See Table 1 for details. For all diabetes models, blood glucose levels were controlled with Linbits administration prior to treatment. Therapies were administered under light isoflurane anesthesia once blood glucose levels were controlled, and prior to Linbits expiring (1–3 weeks post‐implantation). After Linbits expired, no further insulin was given. Unless otherwise indicated, NIs were dosed at 2 × 10e5 NIs/kg b.wt., and administered i.p. suspended in vehicle (0.5 ml serum free DMEM). Where stained or fluorescent cells were administered, omenta, livers, spleens, lungs, kidneys, and pancreata were harvested upon euthanasia and examined by fluorescence microscopy for the presence of fluorescently labeled NIs. Other endpoints are given in Table 1 and Results.
Table 1.
Treatment protocols
| NIs/Clusters composed of | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study question | Recip.mice diabetes type | Recip. strain | Recip. age (wks) | Donor strain/species | Islet cell passage | MSCs passage | Treatment groups | Mice/group | End of study (wks post NI admin.) | Endpoints | ||
| Do NIs reverse hyperglycemia in spont. diabetic mice? | Spont. T1DM | NOD | 13‐21 | C57Bl/6, wt and egfp+ | P1 wt | P5 egfp+ MSCs | NI | 6 | 10 | Sera collected to test for allo Ig‐G response to cells that make up NIs. Omenta examined for T cells. | ||
| NA | NA | NA | Vehicle | 6 | 10 | |||||||
| wt C57Bl/6 | NA | NA | 2x10e5 islets | 3 | 2 | Sera harvested and assessed as above | ||||||
| Are both MSCs and Islet Cells required for clusters to reverse hyperglycemia? | STZ | wt C57Bl/6 | 10 | C57Bl/6, wt and egfp+ | P1 wt | P5 egfp+ MSCs | NI | 6 | 12 (n = 3); 21 (n = 3) | Omenta, pancreata examined by rtPCR for islet associated gene expression | ||
| NA | NA | NA | Vehicle control | 6 | 12 | |||||||
| wt C57Bl/6 | NA | P1 MSCs | MSC only cluster | 5 | 12 | |||||||
| wt C57Bl/6 | P1 wt C57Bl/6 | NA | IC only cluster | 5 | 12 | |||||||
| Do NIs release insulin physiologically or cause hypoglycemia? | Non‐diabetic | wt C57Bl/6 | 12 | C57Bl/6, wt and egfp+ | P1 wt | P5 egfp+ MSCs | NI | 14 | 0.5‐12 | Blood glucose levels, cell tracking | ||
| wt C57Bl/6 | 12 | NA | NA | NA | Vehicle | 3 | 3 | Blood glucose levels | ||||
| NOD/SCID | 9 | Dog | P1 | P2 MSCs | cNI | 6 | 10 | Blood glucose levels | ||||
| NOD/SCID | 9 | NA | NA | NA | Vehicle | 3 | 10 | Blood glucose levels | ||||
| Can NIs derived from canine cells reverse hyperglycemia? | STZ | NOD/SCID | 20 | Dog | P1 | P2 MSCs | cNI; | 5 | 12.5 | Dose finding. Remote onset efficacy. IP GTT at 8 wks, NIs removed at 10 wks. Sera examined for canine specific insulin during IP GTT. | ||
| NA | NA | NA | Vehicle | 5 | 12.5 | |||||||
Abbreviaitons: cNI, canine neo‐islets; egfp+, bears the green fluorescent protein gene, expressed in all cells; IP GTT, intra peritoneal glucose tolerance test; MSCs, mesenchymal stromal cells; NA, not applicable; NI, neo‐islets; admin. administration; NOD, nonobese diabetic; NOD/SCID, nonobese diabetic/severe combined immuno‐deficient; P, Passage number; Recip., recipient; spont., spontaneous; STZ, streptozotocin; wks, weeks; wt, wild type.
In Vivo Imaging
In vivo imaging of DiR (Life Technologies) stained NIs was performed in anesthetized mice using the Li‐Cor, Pearl Impulse imager (LiCor, www.licor.com).
IP Glucose Tolerance Tests
i.p. GTTs were conducted in 3 vehicle‐treated and 5 cNI‐treated STZ‐diabetic NOD/SCID mice by standard procedures (see Supporting Information data), and canine‐ and mouse‐specific insulin levels were assayed by enzyme‐linked immunosorbent assay (ELISA).
Tumor Formation and Ectopic Maldifferentiation of MSCs
Upon euthanasia, all harvested organs were examined histologically for tumors or evidence for ectopic maldifferentiation (osteo‐, adipo‐ or chondrogenic).
Allo‐IgG Response
Aliquots of ∼5×10e4 C57Bl/6 MSCs, ICs, dissociated NIs or dissociated ICs (Accumax) were incubated with 500 μl of serum obtained upon euthanasia from NI‐, vehicle‐, or islet‐treated NOD mice (sera obtained at day 77 for NI and vehicle‐treated, and day 14 for islet‐treated mice), for 30 minutes at room temperature, centrifuged (600g for 5 minutes), incubated with cy3‐conjugated goat‐anti‐mouse IgG antibody (Jackson ImmunoResearch, www.jacksonimmuno.com) or isotype control (1:100 dilution) for 30 minutes fixed, and analyzed by FACS.
Spleen Cell Preparation and T Cell FACS Analysis
Spleens and omenta were sectioned into small pieces, triturated in 1× Phosphate Buffered Saline (PBS, Roche, www.roche.com), passed through a sterile 40 µm strainer (BD) and washed with PBS. Red blood cells were lysed with 1× ACK (Life Technologies) for 10 minutes. Cells were washed with 1× PBS and used directly for FACS staining assays. T and Treg cells were identified using a Mouse T Lymphocyte kit (BD) and a Treg Detection kit (Miltenyi Biotech, www.miltenyibiotec.com). 0.5×10e6 cells were stained per antibody, and 1×10e4 events were counted by FACS (see Supporting Information data).
Statistical Analysis
Data are expressed as Mean ± SEM or Mean ± 95% confidence interval, as indicated. Primary data were collected using Excel (Microsoft, Redmond, WA), and statistical analyses were carried our using Prism (GraphPad, San Diego, California). Two‐tailed t tests and one way ANOVA with Bonferroni Post Test analysis and confidence interval of 95% were used to assess differences between data means. A p value of < .05 was considered significant.
Results
To test our central hypothesis in a clinically informative autoimmune TIDM model, we first examined whether the i.p. administration of in vitro generated allogeneic NIs could reestablish euglycemia in spontaneously diabetic NOD mice as a reflection of (a) their survival, (b) the redifferentiation of ICs contained in the NIs into functional insulin‐producing cells in vivo and re‐expression of other islet‐specific genes, and (c) the MSC‐mediated cyto‐, allo‐ and auto‐immune protection of the transplanted NIs 36, 37, 38, 39, 40, 41, 42, 43. Like humans, NOD mice develop a T‐cell mediated, autoimmune form of T1DM 26, 44, 45.
Formation of NIs
NIs of approximate islet size (150 μm) were prepared as illustrated in Figure 1A. We furthermore confirmed that comparable NIs could be generated from both canine and human ICs and MSCs (Fig. 1B). At 24 hours. post‐formation, NIs remained comprised of approximately 50% MSCs and 50% ICs (Supporting Information Fig. S1).
Figure 1.
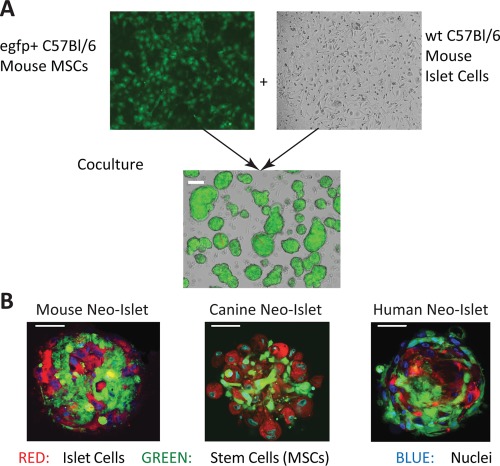
Mouse Neo‐Islets (NI) formation and confocal microscopy. (A): Images (×10) and a schematic representation of mouse cells undergoing NI formation. Approximately 500 green fluorescent protein positive (egfp+) C57Bl/6 MSCs (left, top) and approximately 500 C57Bl/6 islet cells (right, top) were culture expanded, then cocultured in ultra‐low‐adhesion plates where they readily formed NIs overnight (bottom; white scale bar = 150 μm). Average NI‐formation (cells in low adhesion plates incorporated into NIs) efficiency ≥95%; viability ≥ 90%; replicates, N > 25. (B): Confocal microscopic images (×63) of murine (left), canine (middle), and human (right) NIs. Within the NIs, MSCs were Cell Tracker Green stained (green), islet cells were DiI stained (red) and nuclei were stained with DAPI (blue). Morphology and cell composition (approximately 1,000 cells per NI) did not differ significantly among murine, canine, and human NIs. Each depicted NI was approximately 150 μm in diameter. White scale bar = 50 μm. Abbreviation: MSC, mesenchymal stem cell.
Starting Materials for NIs: Growth and Characterization of ICs and MSCs
Upon culture of islets, ICs proliferate and dedifferentiate 12, 13, 14. Upon passaging, IC‐associated gene expression levels decreased. Their gene expression pattern was distinct from that of cultured MSCs (Supporting Information Fig. S2). All ICs were used at P1‐P2 for NI formation. By expanding ICs to P2 and using them at this passage, one canine pancreas, assuming ∼45,000 islets per pancreas, will yield at least 80 therapeutic doses.
All MSCs met the minimal criteria 46, 47, 48 and were used at P1–P5. See Supporting Information data and Supporting Information Figures S3 and S4 for details of epitope expression, trilineage differentiation, INFγ‐induced IDO‐1 expression, role of passage number on gene expression and NI formation, and glucose stimulated insulin secretion by freshly formed NIs.
Treatment of Spontaneously Diabetic NOD Mice with Allogeneic, C57Bl/6 NIs
Allogeneic, C57Bl/6 mouse NIs (2x10e5/kg b.wt., N = 6, see Table 1 and Fig. 1A), 2x10e5 C57Bl/6 mouse islets (N = 3), or vehicle (N = 6) were administered i.p. to spontaneously diabetic NOD mice after blood glucose levels were normalized with slow‐release insulin pellets (Linbits) in order to reduce glucotoxic effects on the transplanted cells 14, 49, 50 and to enhance their in vivo redifferentiation 14, 16, 17. By day 35–40 post‐Linbit treatment, Linbits are depleted, and hyperglycemia redeveloped in both islet‐ and vehicle‐treated NOD mice, as expected 51. Strikingly, blood glucose levels in NI‐treated animals remained near normal (Fig. 2A). These data suggest that (a) the NIs engraft and survive, (b) the ICs within the NIs redifferentiate in vivo, providing the mouse with a new, endogenous source of insulin and potentially other islet hormones, and (c) the MSCs contained in the NIs effectively provide cyto‐protection and allo‐ and auto‐immune‐isolation of the NIs in NOD mice, and together establishing glycemic control in this clinically relevant T1DM model. Next, we explored mechanisms by which this was achieved.
Figure 2.
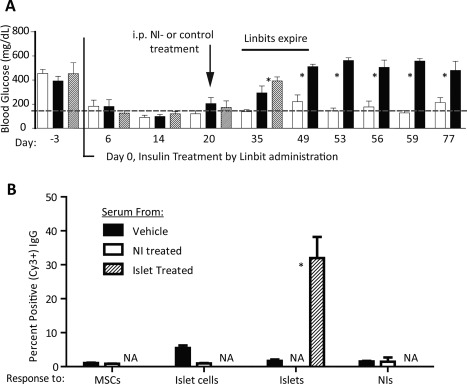
Allogeneic NI‐treatment established euglycemia in spontaneously diabetic nonobese diabetic (NOD) mice without inducing an IgG antibody response. (A): Blood glucose levels (mean ± SEM) of NOD mice normalized with Linbits (day 0), then infused i.p. on day 20 post Linbit therapy with 2×10e5 C57Bl/6 NI/kg b.wt. (N = 6; open bars), vehicle (N = 6; black bars), or 2×10e5 islets (N = 3; hashed bars). While vehicle‐ and islet‐treated mouse blood glucose levels increased when Linbits expired (approximately day 35), euglycemia was maintained long term in NI‐treated mice, implying IC redifferentiation into insulin producing cells and protection from allo‐ and autoimmune attacks. Normal blood glucose level, hashed line. *, p < .05 versus vehicle treated group. (B): IgG responses (FACS; Mean ± SEM) in treated‐NOD mouse sera to C57Bl/6 cells or islets (N = 3 per group). Shown are percentages of cy3+ cells when P5 C57Bl/6 MSCs and P1 C57Bl/6 cultured ICs were incubated with serum and cy3‐labeled anti‐mouse IgG antibody. Sera were from vehicle‐treated and NI‐treated NOD mice from the experiment depicted in (A) collected upon euthanasia (day 77), and from intact C57Bl/6 (allogeneic) islet‐treated NOD mice (positive control) 14 days post i.p. islet administration. Treated‐NOD mice show robust IgG responses to islets, but not to NIs nor to the cells from which NIs are composed. Antibody mediated rejection of NIs appears unlikely since (a) NOD mice remained euglycemic, and (b) FACS data show no IgG response to these cells in otherwise immune competent NOD mice. *, p < .05 versus the other treatments. Abbreviations: NI, neo‐islets; MSC, mesenchymal stem cell; NA, not applicable.
NOD Mice Do Not Mount an Allo‐Immune IgG Response to the MSCs and ICs of NIs
To examine whether ICs and MSCs contained in the NIs are protected from a humoral immune attack 52, we assessed whether sera from normoglycemic, NI‐treated NOD mice contained IgG antibodies directed against either the MSCs or cultured ICs in the NIs. Sera from NI‐treated, normoglycemic NOD mice contained neither IgG antibodies directed at MSCs nor at cultured ICs, while the i.p. administration of identical numbers of allogeneic (C57Bl/6), freshly isolated islets used as a positive control, elicited a robust antibody response (Fig. 2B). The lack of an IgG antibody response to the cells that are used to form the allogeneic NIs, along with the achievement of long term euglycemia, indicates that the NIs also provide humoral, allo‐immune protection to the transplanted cells.
NIs Spontaneously Engraft in the Murine Omentum and Produce Insulin
As shown in Figure 3A, fluorescence in vivo imaging of a euglycemic NOD mouse treated 10 weeks previously with DiR‐labeled, egfp+ NIs demonstrates their persistent location in the upper abdomen. Histological examination upon euthanasia of omenta, pancreata, spleens, livers, lungs, and kidneys from NI‐treated NOD mice from Figure 2A revealed the presence of the egfp+ NI only in the animals' omenta (Fig. 3B). Furthermore, sections of the omentum stained positive for insulin (Fig. 3C left panel), while negative controls (Fig. 3C inset) and omenta from vehicle‐treated, diabetic NOD mice did not (Fig. 3C right panel). Their pancreata showed high‐grade insulitis, as expected (Supporting Information Fig. S5), indicating that euglycemia was achieved through physiologic insulin secretion by the NIs, not islet recovery. Importantly, there was no histologic evidence for tumor formation or ectopic maldifferentiation (adipo‐, osteo‐, chondrogenic) in any examined organs. Additionally, Ki67 staining showed no evidence of proliferation of administered NIs in the omenta.
Figure 3.
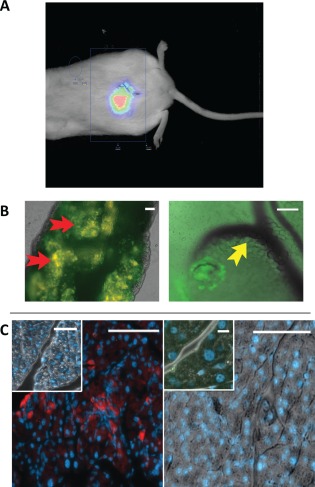
Neo‐Islets (NI) engraftment, survival, and insulin expression in nonobese diabetic (NOD) mice omenta. (A): Fluorescence in vivo imaging of a NOD mouse treated 10 weeks previously with DiR labeled, green fluorescent protein positive (egfp+) NIs demonstrates their location in the upper abdomen. (B): egfp+ C57Bl/6 mouse NIs given i.p. remained engrafted in the omentum and maintained euglycemia in spontaneously diabetic NOD mice at 11 weeks post treatment (see Fig. 2). Left image (×10): representative omentum of a NOD mouse treated with C57Bl/6 egfp+ NIs (green; see red arrows). This image demonstrates that the NIs homed to and engrafted in the omentum, and indicates there is no rejection of the NIs. Right image (×10): enlarged image of a single, engrafted NI. Its location, close to capillaries (yellow arrow) is shown. (C): Left panel, Main image: Sections of the omentum (×10 image) depicted in (B) stained by immunohistochemistry for DNA (Dapi, blue), and insulin protein (red). Insulin protein was clearly detected. Inset, negative control in which the primary, anti‐insulin antibody was omitted. Right panel, Main image: Sections of the omentum (×10) of a vehicle treated, diabetic NOD mouse stained for DNA (blue), and insulin protein (red). Inset: ×40 magnification of the same section (scale bar = 10 μm). No insulin was detected at either magnification. These images demonstrate the omental location and insulin synthesis by engrafted NIs. Except where indicated above, scale bars = 100 μm.
Inhibition of Autoimmune Response
Critical to effectively treating autoimmune T1DM with insulin producing cells is the autoimmune isolation of those cells. The results presented in Figure 2A demonstrate that the ICs within the NIs are protected from NOD mouse autoimmune attack. As in human T1DM, autoimmune beta cell destruction in NOD mice is mediated by autoreactive CD4+ Th1 cells, and is characterized by insulitis involving macrophage, CD4+ and CD8+ T‐cell infiltration 26, 44, 45, 53. It has been shown that allo‐MSC administration either alone 54, 55, 56, 57, 58, 59 or with islets 18, 21, 22, 23, 60, 61 alleviates hyperglycemia in diabetic animals and humans partly by promoting expansion of regulatory T cells and suppressing expansion of immune cells through here confirmed Tgfb1 expression (Supporting Information Figs. S2, S4) and IDO‐1 upregulation (Supporting Information Fig. S3C) 54, 58, 62, 63, 64, 65. To explore the putative immune‐modulating role of NI‐contained MSCs in shielding the NIs from autoimmune attack, we examined a select set of known MSC immunomodulatory mechanisms as follows. Diabetic NOD mice were treated i.p. with allogeneic C57Bl/6 islets (N = 3) or with allogeneic NIs (N = 3). After 14 days, mice were euthanized. Spleens and omenta were harvested and tested by FACS for the percentages of CD3, CD4, CD8, FOXP3, CD25 positive cells. The percent of CD3/CD4 and CD3/CD8 double positive cells (helper and cytotoxic T lymphocytes) were significantly lower in spleen cells of NI‐treated versus Islet treated NOD mice, while the percent of CD4/CD25/Foxp3 triple positive Tregs were significantly increased in the spleens and omenta of NI‐treated versus Islet treated NOD mice (Fig. 4 and Supporting Information Fig. S6A, S6B). While the number of animals tested is small, these results are in agreement with others' findings 54, 55, 56, 58, 60, 61 and with our hypothesis that NIs, and specifically their MSC component, promotes euglycemia in T1 diabetic mice through modulation of the diabetogenic auto‐immune response.
Figure 4.
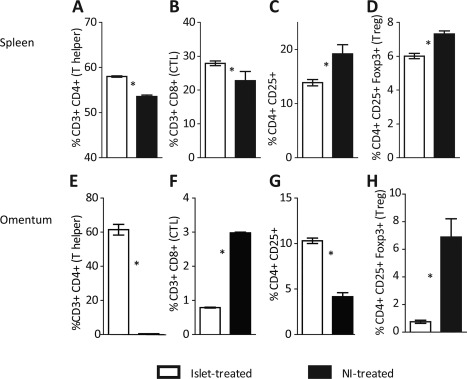
Percent of helper, cytotoxic, and regulatory T (CTL) cells from spleens (A–D) and omenta (E–H) of islet‐treated (N = 3) versus Neo‐Islets (NI)‐treated nonobese diabetic (NOD) mice (N = 3) 14 days post i.p. administration. For (A), (E), (B), and (F), shown are the percent of CD3+ cells that are also (A) and (E) CD4+ or (B) and (F) CD8+. For (C), (G), (D), and (H), shown are the percent of CD4+ cells that were also (C) and (G) CD25+ or (D) and (H) CD25+Foxp3+. While the percentages of helper T cells were lower in NI‐treated mice than in islet treated mice, the percentages of regulatory T cells (Treg) were significantly increased, suggesting that NIs helped restore normoglycemia in NOD mice (see Fig. 2) in part through immune‐modulation. *, p < .05 versus islet‐treated group. Representative FACS histograms of (A) through (H) are shown in Supporting Information Figure 6A and 6B. Abbreviations: CTL, cytotoxic lymphocytes; Treg, regulatory T cells.
Collaboration of ICs and MSCs within NIs Is Essential to Establishing Normoglycemia in Diabetic Animals
To explore the collaboration between ICs and MSCs in NIs, two experiments using a readily controllable Streptozotocin (STZ) model of T1DM in C57Bl/6 mice were conducted and are summarized in Figure 5 (see also Table 1). First, STZ‐diabetic C57Bl/6 mice were treated i.p. with 2×10e5/kg b.wt. syngeneic NIs (N = 6) or with vehicle (N = 6). Second, STZ‐diabetic C57Bl/6 mice were treated i.p. with 2×10e5/kg b.wt. control clusters composed of either MSCs (P1; N = 5) or passaged ICs (P1; N = 5) alone. Importantly, the total number of cells in each generated cell cluster was identical to that in NIs (∼1,000 cells per cluster). Three mice from the NI‐treated group, and all mice from the control groups were euthanized at 12 weeks. The remaining 3 NI‐treated mice were followed for 21 weeks. Long‐term (21 weeks) euglycemia was obtained only in NI treated mice. Treatment with control clusters only minimally reduced blood glucose levels when IC clusters were given (Fig. 5A), demonstrating that both cell types must be present within NIs to facilitate optimal glycemic control.
Figure 5.
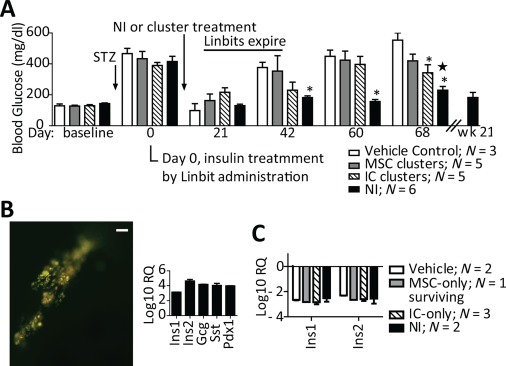
Blood glucose levels of NI versus MSC‐ or IC‐cluster treated, STZ diabetic C57Bl/6 mice and in vivo redifferentiation of ICs into endocrine cells contained in the NIs. (A): Blood glucose levels over time are shown in groups of STZ‐diabetic mice all treated i.p. on day 7 with (i) vehicle, (ii) 2×10e5 MSC clusters/kg b.wt., (iii) 2×10e5 IC clusters/kg b.wt. or (iv) 2×10e5 NIs/kg b.wt. *, p < .05 versus vehicle‐treated group. *, p < .05 versus MSC‐cluster treated group. NIs maintain euglycemia, while MSC‐ and IC‐ clusters do not. (B): Left: Fluorescence image (green, egfp+ cells) of a representative omentum from an NI‐treated, euglycemic mouse 21 weeks post NI injection (scale bar = 200 μm). Right: omental gene expression profile (mean ± SEM) normalized to that of fresh NIs prior to administration, demonstrating NI engraftment, and significant endocrine redifferentiation. (C): Ins1 and Ins2 expression profiles (mean ± SEM) from whole pancreata of MSC‐cluster, IC‐cluster, and NI‐treated versus vehicle‐treated diabetic mice normalized to those of nondiabetic mice. Since pancreatic insulin gene expression levels were similarly decreased in all treatment groups versus those of hyperglycemic, vehicle‐treated mice, it follows that the blood glucose control seen in NI‐treated mice was achieved by insulin secretion from omental NIs. NI + Neo‐Islet. Abbreviations: IC, islet cell; MSC, mesenchymal stromal cell; NI, Neo‐Islets; STZ, Streptozotocin.
In Vivo Redifferentiation
Data from the NOD mouse experiment (Fig. 2), as well as from their retrieved omenta (Fig. 3B) imply that the NIs redifferentiate in vivo to produce sufficient insulin to render mice euglycemic. Indeed, omenta retrieved from the euglycemic, C57Bl/6 NI‐treated mice at 21 weeks showed both engraftment of NIs and significantly increased insulin, glucagon, somatostatin and Pdx1 gene expression compared to freshly formed NIs (Fig. 5B), demonstrating effective in vivo redifferentiation of islet hormone‐expressing ICs. Furthermore, expression of Ins1 and Ins2 in whole pancreata of STZ‐diabetic mice was, as expected, significantly reduced in all animals (Fig. 5C), indicating that euglycemia in NI‐treated mice was achieved by physiological insulin secretion provided by omentally‐engrafted NIs and not by residual pancreatic insulin.
Canine‐Specific Insulin Secretion from cNIs in STZ‐Diabetic NOD/SCID Mice, and Return of Hyperglycemia Upon Their Removal
Spontaneous diabetes mellitus in pet dogs is treated with insulin, but up to 40% are euthanized primarily due to the burden associated with their care 66. Were we to demonstrate, in our Food and Drug Administration approved pilot study (INAD 012776), that cNI therapy was effective in dogs with T1DM, this would reduce euthanasia rates and the burden on dog owners. Furthermore, dogs with T1DM represent a clinically relevant large mammal model, providing potentially valuable information for envisioned clinical trials.
Treatment of NOD/SCID mice (routinely used for xenogeneic protocols) with 2x10e5 cNI/kg b.wt. maintained euglycemia, and significantly, surgical removal of the cNIs from treated, euglycemic mice caused the reappearance of hyperglycemia (Fig. 6A). When this group of mice was subjected to an i.p. GTT (Fig. 6B), only cNI‐treated mice had a normal response, and only these released canine‐specific insulin (Fig. 6C). Taken together, these data demonstrate further that the NIs redifferentiate in vivo to produce and secrete insulin physiologically in response to glucose.
Figure 6.
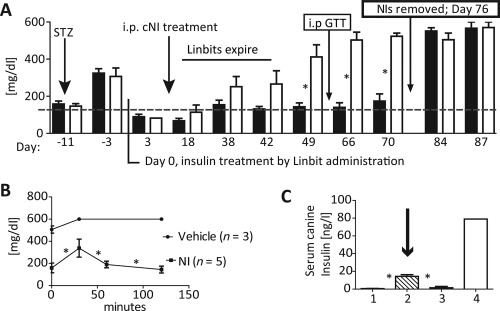
cNIs administered to diabetic nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice redifferentiate in vivo to control blood glucose levels. (A): Blood glucose levels of Streptozotocin diabetic NOD/SCID mice treated with either 2x10e5 cNI/kg b.wt. (black bars, N = 5) or vehicle (open bars, N = 5). cNIs control blood glucose levels long term versus vehicle. Removing cNIs on day 76 resulted in the return of hyperglycemia. (B): IP GTTs on day 63 (arrow) of cNI‐treated NOD/SCID mice were normal versus those of vehicle treated animals. (C): Canine‐specific serum insulin (ELISA) levels rose during the IP GTT only in cNI‐treated (column 2, cross‐hatched bar, arrow, N = 5) NOD/SCID mice. Also shown are canine insulin levels in sera from vehicle‐treated NOD/SCID mice (1st bar, N = 3); nondiabetic C57Bl/6 mice (3rd bar, N = 2, negative control for ELISA specificity) and a nondiabetic dog (4th bar, positive control). Together these data indicate that euglycemia was maintained as a consequence of canine insulin expression and secretion by cNIs. Data: mean ± SEM. *, p < .05 compared to control groups. Abbreviations: cNIs, canine NIs; i.p GTT, intra peritoneal glucose tolerance test; NI, neo‐islets.
Dose Dependency of Glycemic Control, and Control of Remote Onset T1DM by cNIs
To prepare for the canine pilot study, either 8×10e4 or 2×10e5 cNIs/kg b.wt. (cNIs) were administered i.p. to STZ diabetic NOD/SCID mice as indicated in Table 1. cNIs lower blood glucose dose dependently (Supporting Information Fig. S7A). Intraperitoneal administration of 2x10e5 cNI/kg b.wt. to NOD/SCID mice with remote onset STZ‐induced DM, a potential model of therapy initiation later in the course of the disease, similarly restored euglycemia (Supporting Information Fig. S7B).
Intraperitoneal NIs Do Not Cause Hypoglycemia in Nondiabetic Mice
To further ascertain that NIs' insulin delivery is physiologic and does not cause hypoglycemia, nondiabetic C57Bl/6 mice were treated i.p. either with 2x10e5/kg b.wt. syngeneic NIs or vehicle. No animals developed hypoglycemia over time, and blood glucose levels were identical to those in vehicle treated controls (Supporting Information Fig. S8A). Analogous experiments wherein nondiabetic NOD/SCID mice were treated i.p. with either cNIs or vehicle also did not result in hypoglycemia at any time point (Supporting Information Fig. S8B).
Discussion
The present study was designed as a de novo attempt to overcome the principal hurdles that continue to limit the successful treatment of autoimmune‐mediated T1DM with a readily available, progenitor or stem cell based therapy 1, 44. Specifically, our investigations addressed (a) the shortage of suitable pancreas donors for the preparation of adequate islet cell numbers, (b) the difficulty of expanding β‐cells in culture, (c) the permanent need for potentially toxic anti‐rejection drugs in islet and pancreas transplants, (d) the alternative use of encapsulation devices for the immune isolation of islet or progenitor cell transplants, (e) the physiological delivery of insulin into the portal system of the liver, all to be achieved with a minimally invasive mode of NI administration, and by directly harnessing the complex pleiotropic actions of MSCs, cell types that are free of ethical concerns 26, 36, 37, 38, 39, 40, 42, 67, 68, 69. We reasoned that newly formed NIs in which high numbers of healthy MSCs are combined with culture expanded ICs that have undergone expansion and dedifferentiation, would enable these cells, potentially aided by preservation of their epigenetic memory, to re‐differentiate in vivo to functioning β‐cells 12, 14, 70, to survive, and to be shielded from inflammatory and auto‐ and allo‐immune attacks, thereby avoiding the need for anti‐rejection drugs or encapsulation devices. We previously tested the fusion of ICs with MSCs, the creation of heterokaryons, in order to achieve immune protection of the endocrine component of such hybrid cells. We found this approach effective both in vitro and in vivo. However, both the low fusion efficiency and recent reports on the development of malignancies by fused cells made us abandon this technology 71, 72.
Since both standard subcutaneous insulin injections and subcutaneously placed encapsulated endocrine cells deliver insulin not into the portal vein of the liver, where up to 50% of it is inactivated, but exposes peripheral tissues to potentially harmful, supraphysiologic concentrations of this hormone 73, 74, we tested whether the unique biological functions of the well vascularized omentum, that is, the uptake of cells and foreign bodies, could be exploited to incorporate NIs that are intraperitoneally administered. Furthermore, as intrahepatic islet transplants are inefficient, requiring up to 5 donors per often repeated treatment, and being associated in high early losses of islets 1, a successful omental engraftment of NIs would be highly advantageous. If accomplished, it would facilitate their engraftment, redifferentiation and physiological function within the omentum. In this fashion, intraomentally secreted insulin would be, as is physiological, directly delivered into the portal system of the liver. An additional benefit the intraperitoneal location of NIs provides is the fact that glucose sensing from this location is superior to that from the subcutaneous space 29.
There is increasing evidence that MSCs that are located like pericytes in perivascular niches of all blood vessels exert in these microenvironments complex endothelial cell survival, immune‐protective and anti‐inflammatory actions 32, 75, 76. Chronic hyperglycemic states result through various pathomechanisms in microvascular and macrovascular damage, which may also impair various functions of local MSCs and results in what has been termed a “pericytopathy,” best described in the retinal microcirculation 24, 77, 78.
Finally, the novel microenvironment that is created in NIs that are composites of cultured ICs and equal numbers of MSCs, facilitates close range signaling by MSCs through the release of survival factors, cytokines, growth and angiogenic factors, anti‐inflammatory miRNAs, the transfer of mitochondria and beneficial exosomes into adjacent ICs 26, 37, 42, 68, 79, 80, 81, 82. In addition, it would also enable cross talk between insulin producing and other islet endocrine ICs. In contrast to technologies that use monohormonal, that is, insulin‐only producing cells, NIs are expected to contain all or most islet cell types, which may be advantageous. When MSCs are grown in 3D culture, their anti‐inflammatory actions are potentiated, which may further enhance the therapeutic efficacy of the NI technology in subjects with T1DM 83, 84. It is well established that MSCs respond to cues that arise from stressed or damaged cells, resulting in improved survival of such cells and repair through complex paracrine mechanisms, as has been shown in the acutely injured kidney, the bone marrow, and numerous other organs 68, 85. Of further note is the fact that after an NI is formed none of its cells proliferate in vitro or in vivo, and MSCs do not undergo ectopic maldifferentiation or oncogenic transformation. Finally, if indicated by an unanticipated complication, the omentum that harbors NIs can be removed, as we show here, and standard insulin therapy can be resumed.
Although our NI technology appears to be an effective therapy in the tested rodent models, we expect that further refinements or modifications of this therapy will be needed. The i.v. administration of MSCs has been shown to have beneficial effects early in the course of T1DM 23, 26, 55, 56, and may thus be useful as an adjuvant therapy to NIs in subjects with a recent diagnosis of T1DM. The i.p. administration of NIs may be more efficient when delivered in hydrogel, Gelfoam or a thrombin clot, all of which can improve their initial adhesion to the omentum. Should there be evidence for premature rejection and loss of function of cNIs, a short initial course with rapamycin has been reported by others to improve islet survival and function 86. If a potential recipient of this therapy lacks or has a damaged omentum due to a prior intra‐abdominal catastrophe, an intrahepatic transplant or a suitable encapsulation device for i.p. delivery would be required.
Ongoing studies regarding our NI technology are focused on analogous studies using human NIs in diabetic NOD/SCID mice, as well as on the characterization of the NI‐intrinsic microcirculation post engraftment in the omentum, the long‐term distribution of MSCs within the NIs in vivo, their potential differentiation into insulin‐producing and vascular endothelial cells, the redifferentiation of alpha and other endocrine cells in vivo, in situ IDO (canine) and iNOS (murine) expression by MSCs, and a detailed analysis of the long‐term histology and cell composition of functioning NIs.
Conclusion
In conclusion, the data from the present study demonstrate that efficient generation of NIs from mouse, canine and human cells is feasible and reproducible. The i.p. administration of NIs results in their engraftment, redifferentiation and survival in the omentum of spontaneously diabetic NOD mice, in SZT‐diabetic mice, and equally well in allogeneic, syngeneic and xenogeneic treatment protocols. The fact that the therapy of diabetic NOD mice with allogeneic NIs results in durable euglycemia, and absent anti‐islet cell and anti‐MSC antibody production, up regulation of Tregs within the NI‐carrying omentum, demonstrates that the utilized NIs provide both auto‐ and allo‐immune isolation and importantly facilitate redifferentiation of ICs into insulin producing cells. Furthermore, since adequate capillary perfusion is essential for the function of islets in vivo, it follows that the potent angiogenic actions of MSCs induce the formation of a functioning capillary system within the NIs that connects to the omental microvasculature. Similarly, the successful glycemic control in STZ‐diabetic NOD/SCID mice with cNIs provides a strong scientific basis for our Food and Drug Administration approved pilot studies in dogs with T1DM. We expect to generate from these pilot studies additional valuable information for potential future clinical trials.
Finally, the potential benefits NI technology could provide to patients with T2DM lies in the fact that the route of insulin delivery would once again be physiological, and thus would be expected to reduce insulin resistance, insulin‐mediated lipogenesis and potentially harmful exposure of peripheral tissues to high concentrations of subcutaneously administered insulin 44, 45. Respective preclinical studies that investigate these possibilities are currently underway.
Author Contributions
C.W.: conception and design, financial, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript; A.G.: collection and assembly of data, data analysis and interpretation, manuscript writing; J.A. and Z.H.: collection of data; P.Z.: collection and assembly of data.
Disclosure of Potential Conflicts of Interest
A.G., J.A., P.Z., Z.H., and R.H., are employees of SCT, LLC; and C.W. is a consultant to SCT, LLC. C.W., A.G., P.Z., Z.H. are shareholders in SCT, LLC, and declare competing financial interests. Patent pending on the herein described technology.
Supporting information
Supporting Information 1
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Figure 4
Supporting Information Figure 5
Supporting Information Figure 6
Supporting Information Figure 7
Supporting Information Figure 8
Supporting Information Figure 9
Acknowledgments
We thank the following individuals for their encouragement and support of the current studies: A. Gardener, G.R. Reiss, R. Maddock III, A.R. Zander, J. Katz, R. Hansen. This work was funded by SymbioCellTech, LLC. All dog tissues were the generous gift of Dr. Frank Sachse through an NIH sharing agreement.
References
- 1. Barton FB, Rickels MR, Alejandro R et al. Improvement in outcomes of clinical islet transplantation: 1999‐2010. Diabetes Care 2012;35:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polonsky KS. The past 200 years in diabetes. N Engl J Med 2012;367:1332–1340. [DOI] [PubMed] [Google Scholar]
- 3. Lohmann T, List C, Lamesch P et al. Diabetes mellitus and islet cell specific autoimmunity as adverse effects of immunsuppressive therapy by FK506/tacrolimus. Exp Clin Endocrinol Diabetes 2000;108:347–352. [DOI] [PubMed] [Google Scholar]
- 4. Sklavos MM, Bertera S, Tse HM et al. Redox modulation protects islets from transplant‐related injury. Diabetes 2010;59:1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balamurugan AN, Bottino R, Giannoukakis N et al. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas 2006;32:231–243. [DOI] [PubMed] [Google Scholar]
- 6. Zhang N, Su D, Qu S et al. Sirolimus is associated with reduced islet engraftment and impaired β‐cell function. Diabetes 2006;55:2429–2436. [DOI] [PubMed] [Google Scholar]
- 7. Tuch BE, Keogh GW, Williams LJ et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 2009;32:1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weir GC. Islet encapsulation: Advances and obstacles. Diabetologia 2013;56:1458–1461. [DOI] [PubMed] [Google Scholar]
- 9. Ludwig B, Reichel A, Steffen A et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA 2013;110:19054–19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vegas AJ, Veiseh O, Gürtler M et al. Long‐term glycemic control using polymer‐encapsulated human stem cell–derived beta cells in immune‐competent mice. Nat Med 2016;22:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dolgin E. Diabetes: Encapsulating the problem. Nature 2016;540:S60–S62. [DOI] [PubMed] [Google Scholar]
- 12. Joglekar MV, Hardikar A. Epithelial‐to‐mesenchymal transition in pancreatic islet β cells. Cell Cycle 2010;9:4077–4079. [DOI] [PubMed] [Google Scholar]
- 13. Russ H a, Ravassard P, Kerr‐Conte J et al. Epithelial‐mesenchymal transition in cells expanded in vitro from lineage‐traced adult human pancreatic beta cells. PLoS One 2009;4:e6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, York NW, Nichols CG et al. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab 2014;19:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russ HA, Sintov E, Anker‐Kitai L et al. Insulin‐producing cells generated from dedifferentiated human pancreatic beta cells expanded in vitro. PLoS One 2011;6:e25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz TC, Young HY, Agulnick AD et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One 2012;7:e37004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pagliuca FW, Millman JR, Gürtler M et al. Generation of functional human pancreatic β cells in vitro. Cell 2014;159:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duprez IR, Johansson U, Nilsson B et al. Preparatory studies of composite mesenchymal stem cell islets for application in intraportal islet transplantation. Ups J Med Sci 2011;116:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borg DJ, Weigelt M, Wilhelm C et al. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model. Diabetologia 2014;57:522–531. [DOI] [PubMed] [Google Scholar]
- 20. Cavallari G, Olivi E, Bianchi F et al. Mesenchymal stem cells and islet cotransplantation in diabetic rats: Improved islet graft revascularization and function by human adipose tissue‐derived stem cells preconditioned with natural molecules. Cell Transplant 2012;21:2771–2781. [DOI] [PubMed] [Google Scholar]
- 21. Rackham CL, Chagastelles PC, Nardi NB et al. Co‐transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia 2011;54:1127–1135. [DOI] [PubMed] [Google Scholar]
- 22. Rackham CL, Dhadda PK, Le Lay AM et al. Preculturing islets with adipose‐derived mesenchymal stromal cells is an effective strategy for improving transplantation efficiency at the clinically preferred intraportal site. Cell Med 2014;7:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carlsson P‐O, Schwarcz E, Korsgren O et al. Preserved β‐cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015;64:587–592. [DOI] [PubMed] [Google Scholar]
- 24. Hayden MR, Yang Y, Habibi J et al. Pericytopathy: Oxidative stress and impaired cellular longevity in the pancreas and skeletal muscle in metabolic syndrome and type 2 diabetes. Oxid Med Cell Longev 2010;3(5):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther 2016;7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inverardi L, Lanzoni G, Dominguez‐Bendala J et al. MSCs for diabetes. In: Hematti P, Keating A, eds. Mesenchymal Stromal Cells. New York, NY: Springer, 2013:571–597.
- 27. Carlow DA, Gold MR, Ziltener HJ. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J Immunol 2009;183:1155–1165. [DOI] [PubMed] [Google Scholar]
- 28. Litbarg NO, Gudehithlu KP, Sethupathi P et al. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res 2007;328:487–497. [DOI] [PubMed] [Google Scholar]
- 29. Burnett DR, Huyett LM, Zisser HC et al. Glucose sensing in the peritoneal space offers faster kinetics than sensing in the subcutaneous space. Diabetes 2014;63:2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vrabelova D, Adin CA, Kenzig A et al. Evaluation of a high‐yield technique for pancreatic islet isolation from deceased canine donors. Domest Anim Endocrinol 2014;47:119–126. [DOI] [PubMed] [Google Scholar]
- 31. Woolcott OO, Bergman RN, Richey JM et al. Simplified method to isolate highly pure canine pancreatic islets. Pancreas 2012;41:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Traktuev DO, Merfeld‐Clauss S, Li J et al. A population of multipotent CD34‐positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 2008;102:77–85. [DOI] [PubMed] [Google Scholar]
- 33. Tögel F, Isaac J, Hu Z et al. Renal SDF‐1 signals mobilization and homing of CXCR4‐positive cells to the kidney after ischemic injury. Kidney Int 2005;67:1772–1784. [DOI] [PubMed] [Google Scholar]
- 34. Togel F, Weiss K, Yang Y et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. AJP Ren Physiol 2007;292:F1626–F1635. [DOI] [PubMed] [Google Scholar]
- 35. Tögel F, Hu Z, Weiss K et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation‐independent mechanisms. Am J Physiol Renal Physiol; 2005;289:F31–F42. [DOI] [PubMed] [Google Scholar]
- 36. DelaRosa O, Sánchez‐Correa B, Morgado S et al. Human adipose‐derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer‐mediated lysis. Stem Cells Dev 2012;21:1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol 2013;91:19–26. [DOI] [PubMed] [Google Scholar]
- 38. Kim Y‐H, Wee Y‐M, Choi M‐Y et al. Interleukin (IL)‐10 induced by CD11b(+) cells and IL‐10‐activated regulatory T cells play a role in immune modulation of mesenchymal stem cells in rat islet allografts. Mol Med 2011;17:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spaggiari GM, Moretta L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol 2013;91:27–31. [DOI] [PubMed] [Google Scholar]
- 40. LeBlanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol Lett 2015;168:140–146. [DOI] [PubMed] [Google Scholar]
- 41. Plock JA, Schnider JT, Zhang W et al. Adipose‐ and bone marrow–derived mesenchymal stem cells prolong graft survival in vascularized composite allotransplantation. Transplantation 2015;99:1765–1773. [DOI] [PubMed] [Google Scholar]
- 42. Caplan AI, Sorrell JM. The MSC curtain that stops the immune system. Immunol Lett 2015;168:136–139. [DOI] [PubMed] [Google Scholar]
- 43. Ylöstalo JH, Bartosh TJ, Coble K et al. Human mesenchymal stem/stromal cells cultured as spheroids are self‐activated to produce prostaglandin E2 that directs stimulated macrophages into an anti‐inflammatory phenotype. Stem Cells 2012;30:2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eisenbarth GS, Buse JB. Type 1 diabetes mellitus. In: Melmed S, Polonsky KS, Larsen PR et al., eds. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: Elsevier Health Sciences, 2011:1436–1461.
- 45. Brownlee M, Aiello LP, Cooper ME et al. Complications of diabetes mellitus. In: Melmed S, Polonsky KS, Larsen PR et al., eds. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: Elsevier Health Sciences, 2011:1462–1551.
- 46. Ock S‐A, Maeng G‐H, Lee Y‐M et al. Donor‐matched functional and molecular characterization of canine mesenchymal stem cells derived from different origins. Cell Transplant 2013;22:2311–2321. [DOI] [PubMed] [Google Scholar]
- 47. Dominici M, Blanc K Le, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 48. Seo M‐S, Jeong Y‐H, Park J‐R et al. Isolation and characterization of canine umbilical cord blood‐derived mesenchymal stem cells. J Vet Sci 2009;10:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cramer C, Freisinger E, Jones RK et al. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev 2010;19:1875–1884. [DOI] [PubMed] [Google Scholar]
- 50. Tariq M, Masoud MS, Mehmood A et al. Stromal cell derived factor‐1alpha protects stem cell derived insulin‐producing cells from glucotoxicity under high glucose conditions in‐vitro and ameliorates drug induced diabetes in rats. J Transl Med 2013;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Molano RD, Pileggi A, Berney T et al. Long‐term islet allograft survival in nonobese diabetic mice treated with tacrolimus, rapamycin, and anti‐interleukin‐2 antibody. Transplantation 2003;75:1812–1819. [DOI] [PubMed] [Google Scholar]
- 52. Corcione A. Human mesenchymal stem cells modulate B‐cell functions. Blood 2006;107:367–372. [DOI] [PubMed] [Google Scholar]
- 53. Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr Opin Immunol 2014;26:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bassi EJ, Moraes‐Vieira PMM, Moreira‐Sa CSR et al. Immune regulatory properties of allogeneic adipose‐derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 2012;61:2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jurewicz M, Yang S, Augello A et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 2010;59:3139–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Madec AM, Mallone R, Afonso G et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 2009;52:1391–1399. [DOI] [PubMed] [Google Scholar]
- 57. Lee RH, Seo MJ, Reger RL et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA 2006;103:17438–17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fiorina P, Voltarelli J, Zavazava N. Immunological applications of stem cells in type 1 diabetes. Endocr Rev 2011;32:725–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kono TM, Sims EK, Moss DR et al. Human adipose‐derived stromal/stem cells protect against STZ‐induced hyperglycemia: Analysis of hASC‐derived paracrine effectors. Stem Cells 2014;32:1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davis NE, Hamilton D, Fontaine MJ. Harnessing the immunomodulatory and tissue repair properties of mesenchymal stem cells to restore β cell function. Curr Diab Rep 2012;12:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Berman DM, Willman MA, Han D et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 2010;59:2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meisel R, Zibert A, Laryea M et al. Human bone marrow stromal cells inhibit allogeneic T‐cell responses by indoleamine 2, 3‐dioxygenase – mediated tryptophan degradation. Blood 2004;103:4619–4622. [DOI] [PubMed] [Google Scholar]
- 63. Su J, Chen X, Huang Y et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell‐mediated immunosuppression in mammalian species. Cell Death Differ 2014;21:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kang JW, Kang K‐S, Koo HC et al. Soluble factors–Mediated immunomodulatory effects of canine adipose tissue–derived mesenchymal stem cells. Stem Cells Dev 2008;17:681–694. [DOI] [PubMed] [Google Scholar]
- 65. Spaggiari GM, Capobianco A, Abdelrazik H et al. Mesenchymal stem cells inhibit natural killer‐cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3‐dioxygenase and prostaglandin E2. Blood 2007;111:1327–1333. [DOI] [PubMed] [Google Scholar]
- 66. Fall T, Hamlin HH, Hedhammar A et al. Diabetes mellitus in a population of 180,000 insured dogs: Incidence, survival, and breed distribution. J Vet Intern Med 2007;21:1209–1216. [DOI] [PubMed] [Google Scholar]
- 67. Burr SP, Dazzi F, Garden O a. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol 2013;91:12–18. [DOI] [PubMed] [Google Scholar]
- 68. Caplan AI. Adult mesenchymal stem cells: When, where, and how. Stem Cells Int 2015;2015:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Davis TA, Anam K, Lazdun Y et al. Adipose‐derived stromal cells promote allograft tolerance induction. Stem Cells Transl Med 2014;3:1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bramswig NC, Kaestner KH. Epigenetics and diabetes treatment: An unrealized promise? Trends Endocrinol Metab 2012;23:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou X, Merchak K, Lee W et al. Cell fusion connects oncogenesis with tumor evolution. Am J Pathol 2015;185:2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Azizi Z, Lange C, Paroni F et al. β‐MSCs: Successful fusion of MSCs with β‐cells results in a β‐cell like phenotype. Oncotarget; 2016;7:48963–48977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gregory JM, Kraft G, Scott MF et al. Insulin delivery into the peripheral circulation: A key contributor to hypoglycemia in type 1 diabetes. Diabetes 2015;64:3439–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pørksen N, Grøfte T, Greisen J et al. Human insulin release processes measured by intraportal sampling. Am J Physiol Endocrinol Metab 2002;282:E695–E702. [DOI] [PubMed] [Google Scholar]
- 75. Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 76. Humphreys BD. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol 2012;32:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wisniewska‐Kruk J, Klaassen I, Vogels IMC et al. Molecular analysis of blood–retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp Eye Res 2014;122:123–131. [DOI] [PubMed] [Google Scholar]
- 78. Hammes H‐P. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 2005;37(suppl 1):39–43. [DOI] [PubMed] [Google Scholar]
- 79. Boumaza I, Srinivasan S, Witt WT et al. Autologous bone marrow‐derived rat mesenchymal stem cells promote PDX‐1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun 2009;32:33–42. [DOI] [PubMed] [Google Scholar]
- 80. Spees JL, Olson SD, Whitney MJ et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 2006;103:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Islam MN, Das SR, Emin MT et al. Mitochondrial transfer from bone‐marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang B, Yin Y, Lai RC et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 2014;23:1233–1244. [DOI] [PubMed] [Google Scholar]
- 83. Bartosh TJ, Ylostalo JH, Mohammadipoor A et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA 2010;107:13724–13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bartosh TJ, Ylöstalo JH, Bazhanov N et al. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self‐activates caspase‐dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells 2013;31:2443–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tögel FE, Westenfelder C. Mesenchymal stem cells: A new therapeutic tool for AKI. Nat Rev Nephrol 2010;6:179–183. [DOI] [PubMed] [Google Scholar]
- 86. Cheng P‐P, Liu X‐C, Ma P‐F et al. iPSC‐MSCs combined with low‐dose rapamycin induced islet allograft tolerance through suppressing Th1 and enhancing regulatory T‐cell differentiation. Stem Cells Dev 2015;24:1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Figure 4
Supporting Information Figure 5
Supporting Information Figure 6
Supporting Information Figure 7
Supporting Information Figure 8
Supporting Information Figure 9


