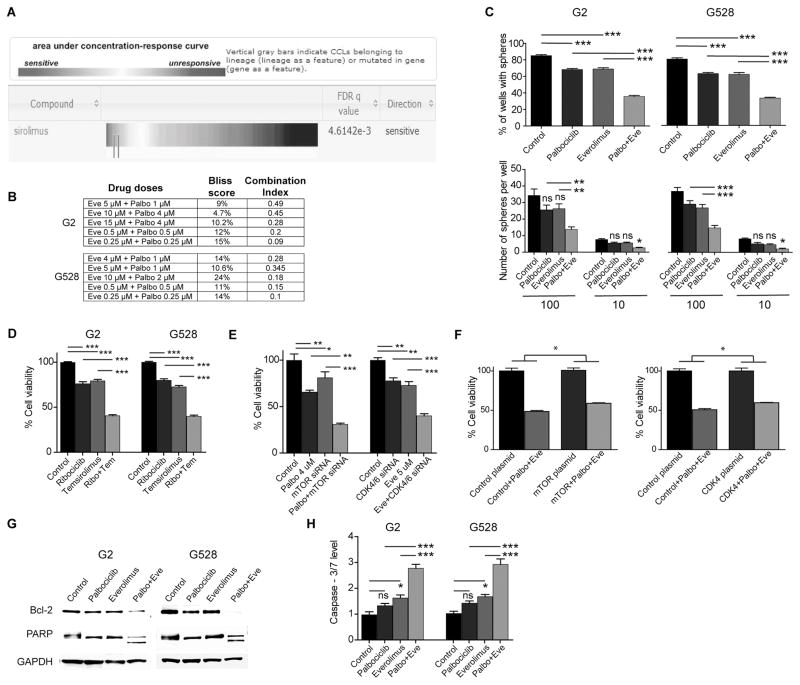
Figure 1. The combination of CDK4/6 and mTOR inhibition is synergistic against GBM.
(A) Enrichment analysis showing increased sensitivity of cancer lines with mutations in RB1 to an mTOR inhibitor, sirolimus. (B) Synergy scores of the combination of CDK4/6 and mTOR inhibition in two GIC lines calculated with both the Bliss and the Chou-Talalay methods. (C) 10 and 100 GICs were cultured in 24-well plates over two weeks to compare sphere formation upon treatment with vehicle, palbociclib (1 μM), everolimus (4 μM), and the combination of palbociclib and everolimus (*P < 0.05; **P < 0.001; ***P < 0.0001; one-way analysis of variance (ANOVA) with post-hoc Tukey analysis). (D) The combination of a different mTOR inhibitor temsirolimus (4 μM) and a different CDK4/6 inhibitor ribociclib (4 μM) is also synergistic against GICs (***P < 0.0001; one-way ANOVA with post-hoc Tukey analysis). (E) The combinations of palbociclib (4 μM) with mTOR siRNA and everolimus (5 μM) with CDK4/6 siRNA are also synergistic against GICs (each treatment line received either DMSO or the indicated drug and either control siRNA or a specific siRNA) (**P < 0.001; ***P < 0.0001; one-way ANOVA with post-hoc Tukey analysis). (F) Constitutively-active CDK4 and mTOR plasmids partially rescued from the effects of the combination treatment (each treatment line received either a control plasmid or the indicated active plasmid and either DMSO or the combination treatment) (*P < 0.05; two-tailed t-test). (G and H) Combined CDK4/6 and mTOR inhibition induces significant apoptosis. Shown is an immunoblot using antibodies specific for Bcl-2 and PARP. Caspase-3/7 level is increased with the three days of combined treatment. (*P < 0.05; ***P < 0.0001; one-way ANOVA with post-hoc Tukey analysis).
Eve: Everolimus, Palbo: Palbociclib, Ribo: Ribociclib, Tem: Temsirolimus, NS: Non-Significant. All values are mean ± SEM of triplicates. Cell viabilities were determined via cell counts following 3 days of treatment. Each experiment was performed 3 times using separate samples.