Abstract
Malignant mesothelioma (MM) is an aggressive malignancy, highly resistant to current medical and surgical therapies, whose tumor cells characteristically show a high level of aneuploidy and genomic instability. We tested our hypothesis that targeting chromosomal instability in MM would improve response to therapy.
TTK/Mps-1 (monopolar spindle 1 kinase) is a kinase of the spindle assembly checkpoint that controls cell division and cell fate. CFI-402257 is a novel, selective inhibitor of Mps-1 with antineoplastic activity. We found that CFI-402257 suppresses MM growth. We found that Mps-1 is overexpressed in MM and that its expression correlates with poor patients’ outcome. In vitro, CFI-402257-mediated inhibition of Mps-1 resulted in abrogation of the mitotic checkpoint, premature progression through mitosis, marked aneuploidy and mitotic catastrophe. In vivo, CFI-402257 reduced MM growth in an orthotopic, syngeneic model, when used as a single agent, and more so when used in combination with cisplatin+pemetrexed, the current standard of care.
Our preclinical findings indicate that CFI-402257 is a promising novel therapeutic agent to improve the efficacy of the current chemotherapeutic regimens for MM patients.
Keywords: Mesothelioma, TTK, Mps-1, CFI-402257, genomic instability
Introduction
Malignant mesothelioma (MM) is an aggressive cancer originating from the linings of the pleural, peritoneal and pericardial cavities. Epidemiologically, MM is associated to exposure to carcinogenic mineral fibers, such as asbestos. MM causes about 40,000 deaths per year worldwide and is characterized by a median survival of about 1 year.1–4 The standard first-line therapy for MM is the combination of a platinum derivative (e.g. cisplatin) and an antimetabolite drug (e.g. pemetrexed).1, 5 However, chemotherapy only marginally impacts patients’ survival because of MM intrinsic chemo-resistance and polyclonal origin which is related to the carcinogenic field effect of asbestos.6, 7 Therefore, there is an urgent need to identify novel, more effective therapies.
Unlike lung cancer and other adult malignancies caused by exposure to carcinogens, MM has a very low average number of mutations, range 2–52, similar to pediatric malignancies.8 Moreover, the few recurrently mutated driver genes in MM are tumor suppressor genes, BAP1, NF2, CDKN2A, SETD2, PBRM1, while activating mutations of oncogenes are rare.8–13 Instead, larger chromosomal aberrations and copy number variations,14–17 as well as non-contiguous minute deletions,18 are commonly found in MM. These findings suggest that chromothripsis and widespread chromosomal instability are much more relevant in MM pathogenesis than nucleotide level activating mutations. These findings perfectly fit within a model of MM pathogenesis, caused by the concurrent activities of asbestos-induced DNA damage,19 and inflammation,20–22 acting in concert with genetic susceptibility and instability,20, 23, 24 and possibly other cofactors.25, 26 Very recently, the mechanisms of gene-environment interaction that lead to MM have been elucidated27, 28
We speculated that MM would develop adaptive mechanisms to tolerate genomic instability and aneuploidy, as seen in some other cancer types,29, 30 and that these mechanisms could be exploited therapeutically. The spindle assembly checkpoint (SAC) is the main mechanism that maintains chromosomal stability through the cell division.31–33 TTK, also known as Monopolar Spindle 1 (Mps-1) is a dual-specificity, serine threonine kinase, and a critical SAC component. Mps-1 promotes the formation of the mitotic checkpoint complex,31, 34 regulates proper alignment of chromosomes,35 cytokinesis,36 chromosome duplication37 and the DNA damage response.38 Mps-1 is overexpressed in several cancer types, as high levels of Mps-1 facilitate the survival of tumor cells with aneuploidy.39, 40 In contrast, reduction of Mps-1 levels triggers SAC loss and elicits massive mis-segregation of chromosomes, which leads to cell death.41–44 Recently, Mps-1 emerged as a potential therapeutic target in cancer, especially in combination with chemotherapy.43, 45, 46
Results and discussion
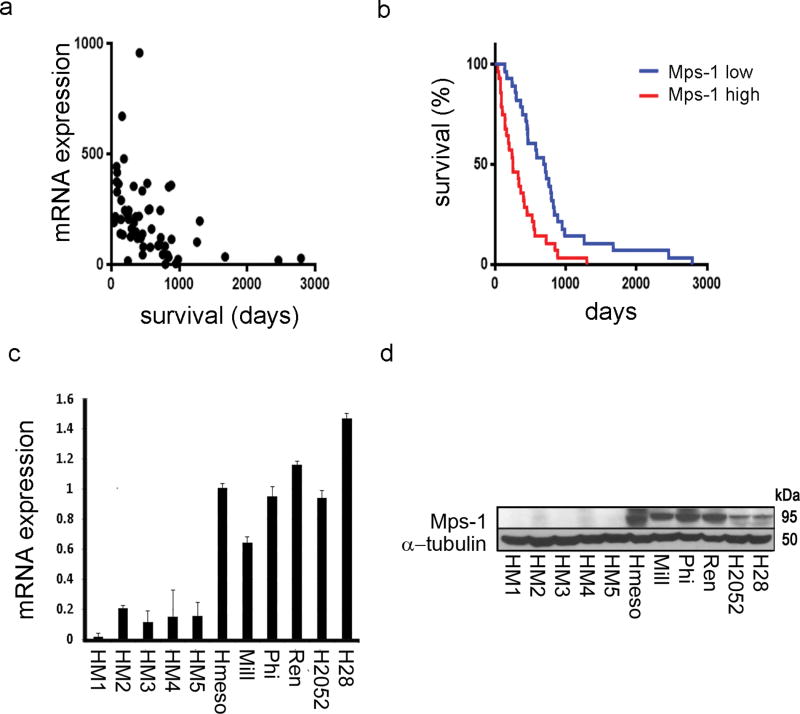
We used a publicly available TCGA database47 to analyze survival data of 56 MM patients for the correlation between Mps-1 mRNA expression levels and the time of death from initial MM diagnosis. Mps-1 mRNA levels significantly correlated with a shorter patients’ survival (r = −0,41; 95%CI −0.61 to −0.17; P < 0.01) (Figure 1a). We dichotomized cases in Mps-1high and Mps-1low, based on whether mRNA levels were above or below the median value, and analyzed survival comparing Kaplan-Meir curves with log-rank test. Mps-1high MM cases had significantly shorter survival compared to Mps-1low MM cases (median survival 254 days vs. 699 days; HR 0.42; 95%CI 0.24 to 0.75; P < 0.001) (Figure 1b). Analysis of the TCGA dataset, also uncovered that Mps-1 mRNA expression was significantly higher in non-epithelioid (non-E) histologic subtypes (biphasic, sarcomatoid and diffuse MM –as per TCGA classification), as compared to the epithelioid subtype (Sup. Info. 1). Conversely, no correlation between Mps-1 mRNA expression and different tumor stages was found (Sup. Info. 1). Overall, because non-E MMs are more aggressive and have a worse prognosis,48 the higher Mps-1 expression in the non-E could, at least in part, explain the association between high Mps-1 expression and poor survival. Using the TCGA mesothelioma dataset, a comparison of mRNA and Copy Number Variation indicated that overexpression of Mps-1 mRNA (i.e. highest quartile), was significantly more common in MMs harboring CDKN2A homozygous or heterozygous deletions (Log Odds Ratio = 2.33, P < 0.001), while no statistical association was found between Mps-1 mRNA overexpression and deletions in the other commonly altered MM tumor suppressor genes, BAP1 and NF2. Next, we compared Mps-1 mRNA and protein levels from a panel of MM lines and from human mesothelial primary cell cultures (HM) that we routinely establish from pleural fluids of patients with nonmalignant conditions.49 Mps-1 expression was consistently and significantly higher among all MM cell lines, compared to HM tissue cultures (1.03 ± 0.25 vs 0.13 ± 0.06, P = 0.043) (Figure 1c). In line with the mRNA data, protein levels of Mps-1 were higher in MM cell lines (Figure 1d). Together, these data supported the hypothesis that Mps-1 might be critical for the development of MM and that it could represent a useful novel therapeutic target.
Fig.1. Mps-1 expression levels correlate with MM malignancy.
(a,b) Mps-1 mRNA levels correlate with survival in MM patients. (a) Mps-1 mRNA expression levels (log2) from MM tumors plotted against time of patients’ survival after diagnosis. (b) Kaplan-Meier survival curve for overall survival of patients with low and high expression of Mps-1. mRNA expression data was obtained from the cBioPortal for Cancer Genomics dataset. On the basis of median Mps-1 expression, patients were classified as Mps-1 high (median > 178) and Mps-1 low (median < 178). The curve indicates a statistically significant reduction in overall survival with higher Mps-1 mRNA expression (p = 0.0001). (c,d) Mps-1 mRNA and protein levels correlate with MM phenotype. (c) Mps-1 mRNA levels were detected by qRT-PCR in a panel of MM cell lines and HM cell cultures, using SYBR Green MasterMix (Applied Biosystems, Foster City, CA, US) on 7900HT Fast Real Time PCR System (Applied Biosystems). The following primer pairs were used; Mps-1: For:5′-TGGCCAACCTGCCTGTTT-3′; Rev:5′-AATGCATTCATTTGCTGAAGAAGA-3′. (d) Total protein lysates from a panel of MM cell lines and HM cell cultures were immunoblotted and stained with anti-TTK (#3255, Cell Signaling, US) and anti-α-tubulin as a loading marker.
The initial effect of SAC abrogation, through inactivation of Mps-1, is manifested by an accelerated entry into a mitotic process, which can be assessed by measuring the phosphorylation of histone H3 on Ser10 and Ser28 residues (phospho-H3).50 This process eventually results in exaggerated aneuploidy, mitotic catastrophe and cell death.43 We, therefore, evaluated the effects of Mps-1 inhibition in MM cells using CFI-402257, a recently discovered, specific and potent small molecule inhibitor of Mps-1.46, 51
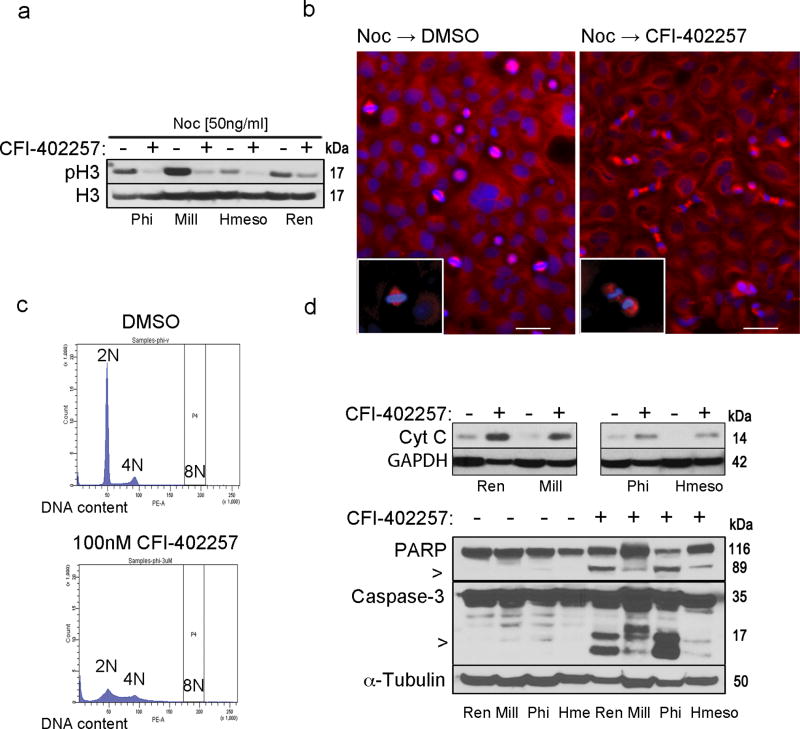
Western blot analyses showed reduced levels of phospho-H3 in CFI-402257-treated compared to DMSO-treated MM cell lines, after release from nocodazole-induced G2/M arrest (Figure 2a). The result was confirmed in a microscopy time-course analysis (Figure 2b). These findings are consistent with reduced SAC function and accelerated progression through the cell cycle. Next, we studied the impact of Mps-1 inhibition with CFI-402257 on the first and the second round of division of MM cells. Treatment with CFI-402257 was associated with increased frequency of errors, such as misaligned and lagging chromosomes, observed in meta- and anaphase, inter-nuclear bridging in telophase or unequal chromosome separation, and consequent unequal division, multilobulation and formation of micronucleus (Table 1, Sup. Info. 2). In addition, CFI-402257-induced perturbations in cell cycle, led to an increase in the proportion of aneuploid cells, manifested as a widening of the peaks in cytofluorometric analysis of DNA content (Figure 2c). No significant polyploidy was observed with concentration as high as 3 µM, which agrees with previous reports46 and confirms the selectivity of the compound (Sup. Info. 3). The massive aneuploidy observed, should not be tolerated. Accordingly, CFI-402257-treated MM cells ultimately underwent cell death with features characteristic of mitotic catastrophe,52 as shown by cytoplasmic translocation of the cytochrome c (Cyt C) (Figure 2d upper panel) and cleavage of caspase-3 and PARP (Figure 2d lower panel). Together our results showed that CFI-402257 leads MM cells to exit mitosis with mis-segregated chromosomes, resulting in exaggerated aneuploidy and consequent cell death.
Fig.2. CFI-402257 induces mitotic checkpoint bypass, defects in mitotic division and mitochondrial death of MM cells.
Ren, Mill, Phi and Hmeso cells were treated with 50 ng/ml nocodazole (Noc) (Sigma-Aldrich, MO, US) for 10 hr and then released from the G2/M arrest in full media containing 100 nM CFI-402257 or DMSO. (a) After 3 hr cells were collected and the immunoblot analysis of total cell lysates was performed with phospho-H3 and total H3 antibody as the loading control. Picture is a representative of two independent experiments performed with pSer10H3 and pSer28H3 (#9701 and #9713, Cell Signaling, US) antibodies. (b) After 30 min cells were stained for mitotic spindle (α-tubulin: T6199, Sigma-Aldrich, MO, US) and nuclei (DAPI). Picture magnification is 10×. Bar: 200 µm. Insets show 40× magnification of the cell in metaphase and telophase (boxed regions). (c) Phi cells were treated with 100 nM CFI-402257 or DMSO for 72 hr, and then ethanol-fixed and stained with propidium iodide for the cytofluorometric analysis of DNA content. Data are representative cell cycle histograms. Experiment was repeated in other MM cell lines with similar results. (d) Phi and Hmeso cells were treated with 100 nM CFI-402257 or DMSO. To assess Cyt c release (top panel) after 72 hr of treatment, fractionation was performed by incubation of harvested cells in lysis buffer (20 mM HEPES-KOH pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM bisodium EDTA, 1 mM EGTA, sucrose) and aspiration through a 22-gauge needle followed by centrifugation at 1000g for 5min at 4C to separate nuclei. The supernatant was centrifuged at 10 000g for 20 min in 4C to separate mitochondria. The cytoplasmic fraction was immunoblotted and Cyt c (#556432, BD, CA, US) was detected. After 5 days of treatment, total lysates of Ren, Mill, Phi and Hmeso were collected and immunoblotted. Caspase-3 and PARP (#9662 and #9542, Cell Signaling, US) were detected (bottom panel), α-tubulin and GAPDH served as a loading control. All the data are representative of two independent experiments. For space constraints, ‘Hme’ stands for ‘Hmeso’ MM cell line.
Table 1.
Treatment with CFI-402257 causes premature progression through the mitotic division, resulting in accumulation of mitotic aberrations in MM cells.
| Cell line | Condition | cells in meta-/ana-phase (%) | cells in telophase (%) | division errors (%) |
|---|---|---|---|---|
| Phi | Vehicle | 10.85 ± 1.65 | 0.43 ± 0.35 | 8.00 |
| CFI-402257 | 4.89 ± 1.55 | 9.92 ± 2.29 | 34.23 | |
| Hmeso | Vehicle | 10.83 ± 2.99 | 0.33 ± 0.62 | 10.35 |
| CFI-402257 | 4.28 ± 1.58 | 8.32 ± 2.39 | 43.36 |
Phi and Hmeso cells (105 cells/slide chamber) were pretreated with 50 ng/mL nocodazole for 10 hr to induce G2/M arrest and then released in full media with CFI-402257 or DMSO for 30 min. Cells were stained for mitotic spindle (α-tubulin) and nuclei (DAPI). First, cells in metaphase/early anaphase and telophase were counted. A total of 1400 cells (Phi) and 800 cells (Hmeso) were examined per treatment condition. Next, microscopic analysis of dividing cells was performed and cells exhibiting errors such as lagging chromosomes and unequal division were counted out of 50 cells in telophase. Two independent experiments were performed. Mean ± SD were calculated with Student’s t test.
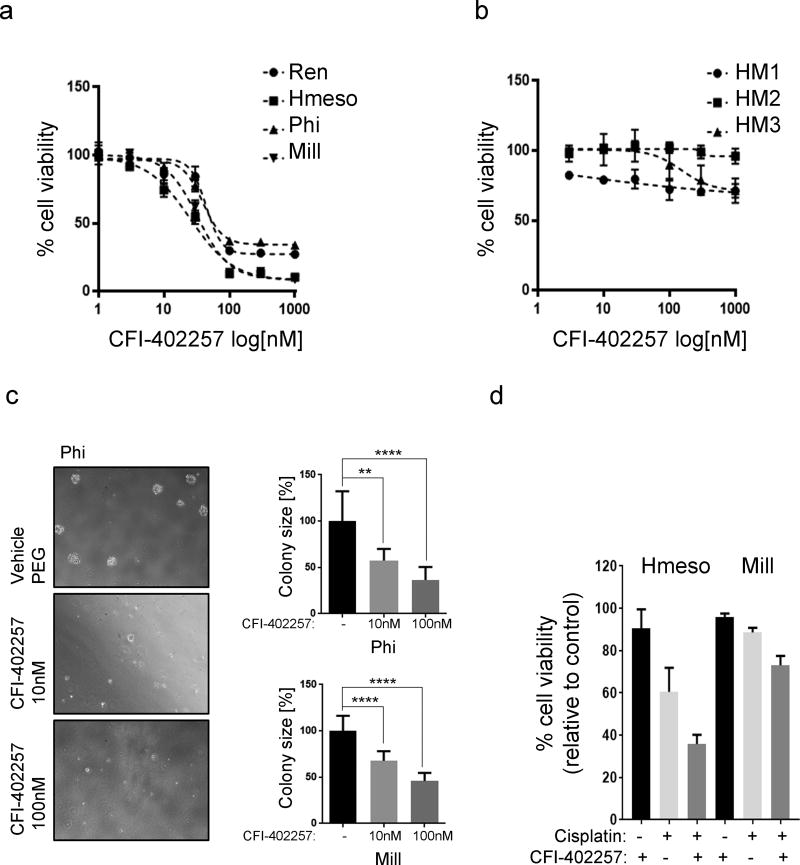
Our observations were paralleled by the specific effect of CFI-402257 on MM cells viability, with EC50 values ranging between 20–40 nM (Figure 3a), whereas three normal primary HM cultures (from different donors) were largely unaffected at these same concentrations (Figure 3b). Moreover, treatment with CFI-402257 showed a tendency to decrease the number of MM colonies in a soft agar assay (Sup. Info. 4), which closely mimics tridimensional tumor growth, and CFI-402257 significantly reduced colony size (Mill: 67.8% (10 nM) and 46% (100 nM), P < 0.0001; Phi: 57.4% (10 nM), P = 0.0016 and 36.4% (100 nM), P < 0.0001) (Figure 3c).
Fig.3. CFI-402257 suppresses growth of MM cells in vitro and has no effect on normal mesothelial cells.
Five human MM cell lines (a) and three primary cultures of mesothelial cells derived from non-cancer patients (b) were plated 3 × 103 cells/well of 96-well plate and treated with increasing concentrations of CFI-402257. Alamar Blue (Thermo Fisher, MA, US) viability assay was performed after 5 days of treatment. EC50 values were calculated using GraphPad PRISM software. (c) The ability of MM cell lines to form colony in soft agar was evaluated under treatment with 10 nM and 100 nM CFI-402257 or DMSO. Total of 20 (Phi) and 10 (Mill) colonies were measured from 3 wells per condition. The graph represents average colony size expressed as the percentage of vehicle. Pictures are representative of the experiment performed with Phi cells. (d) Mill and Hmeso cell lines were plated 3 × 103 cells/well of 96-well plate and treated with 10µM Cisplatin and/or 10nM CFI-402257. After 72 hr, Alamar Blue viability assay was performed. To determine the effects of drug combinations CDI was calculated58 as follows: CDI = AB/(A × B), where AB is the ratio of the measured effect of combination to control group; A or B is the ratio of the single agent to control group. Thus, CDI <1, = 1 or >1 indicates that the drugs are synergistic, additive or antagonistic, respectively. CDI <0.7 indicates that the drug is significantly synergistic.
Cisplatin+pemetrexed is the current first-line standard of care for MM, and it is associated with a modest average extended survival of about 11 weeks.5 We hypothesized that Mps-1 inhibition could synergize with cisplatin-induced cell death. We found that CFI-402257 and cisplatin have a synergistic effect –combined effect of the coefficient of drug interaction (CDI) value below 1, in vitro, in both Hmeso, a relatively cisplatium-sensitive MM line, and Mill, a relatively cisplatin-resistant line (CDI=0.652 for Hmeso; CDI=0.86 for Mill) (Figure 3d).
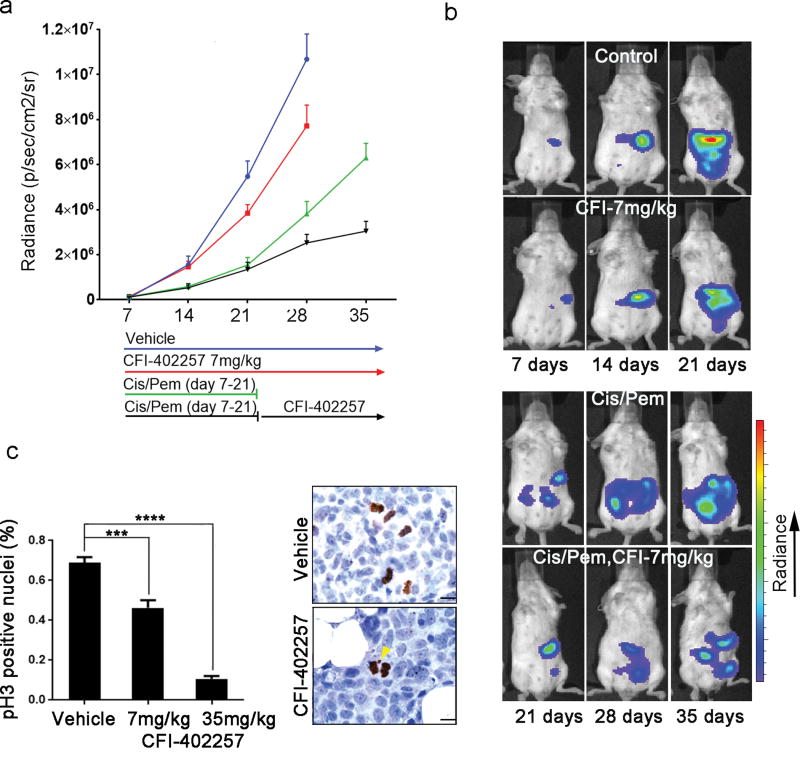
We tested the efficacy of CFI-402257 in an orthotopic, syngeneic model of MM, using the well-characterized AB12 murine MM cell line.53 Similar to human MM cells, AB12 in vitro growth was inhibited by CFI-402257 at nanomolar concentrations (Sup. Info. 5a). Mice were injected intra-peritoneally (i.p.) with AB12 cells carrying luciferase gene. Six days later, following the establishment of the tumors, as detected by IVIS imaging, mice were randomized to receive 1) vehicle (PEG), 2) CFI-402257 alone (7 mg/kg), 3) cisplatin+pemetrexed –(Cis/Pem), for 2 weeks followed by CFI-402257 till study end-point or 4) Cis/Pem, discontinued after 2 weeks, as a control (Figure 4a). The Cis/Pem-CFI-402257 combination regimen adopted in our in vivo study, was chosen to mimic the approach of approval process for the use of new drugs in clinic. Treatments were well tolerated, as we did not observe overt toxicity or any weight loss upon CFI-402257 treatment as single agent. Less than 10% of body mass loss was observed in the Cis/Pem-CFI-402257 regimen group (Sup. Info. 5b).
Fig.4. CFI-402257 significantly reduces MM tumor growth in vivo, alone and in combination with chemotherapy.
Forty BALB/c mice were injected i.p. with 5 × 105 AB12 cells carrying luciferase gene. Six days later, detectable tumor nodules were verified by IVIS imaging and the mice were randomized into the following treatment regimens (n=10 mice/group): 1) vehicle-polyethylene glycol (PEG); 2) CFI-402257; 3) Cis/Pem (days 7–21) followed by PEG and 4) Cis/Pem (days 7–21) followed by CFI-402257. CFI-402257 was administered through oral gavage, at the dose of 7 mg/kg, daily. Cis/Pem (Sigma-Aldrich, MO, US) were injected i.p., with the following treatment scheme: Cis: 1 mg/kg, 1/week, Pem: 50 mg/kg, 3/week. IVIS detection of the growing tumors was performed once a week. (a) The kinetics of tumor growth. (b) Representative IVIS images. (c) Six BALB/c mice were injected i.p. with 5 × 105 AB12-luc cells. Tumors were allowed to develop for 15 days then mice were randomized into vehicle (PEG), CFI-402257 (7 mg/kg), and CFI-402257 (35 mg/kg). CFI-402257 was administered through oral gavage twice a day, for 5 days. On day 6 mice were sacrificed and tumors were collected. Graph shows the mean ± SEM of the percentage of phospho-H3 (S10) positive nuclei counted from 20 images taken from each tumor tissue. ***P < 0.01, ****P < 0.0001. P values were calculated using Student’s t test. Picture shows staining with phospho-H3 (S10) antibody depicting (yellow arrow) chromosome loss during the cell division in CFI-402257-treated tumor tissue. Bar; 10 µm.
To compare tumor growth rates among the four treatment groups, we ran mixed (repeated observations over time) polynomial regression analyses, which accounted for nonlinear growth. The outcome was tumor radiance and the predictors were groups, days of growth, and the square of days. To assess group differences in growth rate, the interaction term between group and days was included, providing a slope comparison test. Tumor size was assumed to be zero at zero days. The Mixed procedure in the SAS 9.4 software (SAS Institute Inc., Cary, NC) performed the analyses. Kaplan-Meier survival curves were compared using the log-rank (Mantel-Cox) test using GraphPad Prism 7.0 (GraphPad Software).
We found that CFI-402257 had moderate antitumor effect, as a single agent, and significant effect when added to the standard chemotherapy regimen. Specifically, we found that CFI-402257 treatment alone significantly reduced tumor growth compared to vehicle treatment [t(51) = −3.6, P = 0.0008], even though to a lesser extent compared to standard chemotherapy. More importantly, CFI-402257 synergized with chemotherapy to significantly delay tumor growth, after cessation of standard chemotherapy [t(71) = 4.9, P < 0.0001] (Figure 4a, b). Reduction in tumor growth resulted in significantly prolonged overall survival in treated mice in both CFI-402257 treatment alone [χ2(1) = 15.9, P < 0.0001] and Cis/Pem-CFI-402257 group [χ2(1) = 8.4, P = 0.004] (Sup. Info. 5c, d).
Short-term in vivo experiment to study the mechanism of CFI-402257 in vivo revealed evidence of mitotic errors in the tumor tissue harvested from CFI-402257-treated animals (Figure 4c). Quantification of phospho-H3 (S10) positive nuclei, revealed a reduction of the mitotic index in CFI-402257-treated tumors (0.68% for vehicle vs 0.46% for 7mg/kg dose, P = 0.0001 and 0.1% for 35 mg/kg dose, P < 0.0001) (Figure 4c).
The low mutational load in MM8 and its frequent polyclonal origin,7 provide a rationale for the observation that targeted therapies for MM have been ineffective. Accordingly, the prognosis and the median survival of MM have depressingly remained almost unchanged over the past several decades. We reasoned that new therapeutic strategies should aim at targeting common mechanisms required for survival of all tumor cells, rather than uncommon activating mutations of oncogenes in MM, which are present only in some but not all tumor cells. Genome instability in the context of cancer cells is considered an example of hormesis, a process that exhibits a biphasic response, with beneficial effects at low levels, but lethal at higher levels.45, 54 Hence, exacerbation of aneuploidy targeting SAC components eventually cannot be tolerated. For this reason, mitotic kinases are now considered effective ‘druggable’ targets in cancer therapy. The chemistry and pharmacology of kinase inhibitors have developed over the last two decades to enable oral administration.55 CFI-402257 or N-cyclopropyl-4-(7-((((1s,3s)-3-hydroxy-3-methylcyclobutyl)methyl)amino)-5-(pyridin-2-yloxy)pyrazolo[1,5-a]pyridin-3-yl)-2-methylbenzamide, belongs to the novel class of pyrazolo[1,5-a]pyrimidine Mps-1/TTK inhibitors. It was identified and developed through the drug discovery program of the Campbell Family Institute for Breast Cancer Research. CFI-402257 optimization of its physicochemical and pharmacokinetic properties led to a potent Mps-1/TTK inhibitor (Ki=0.1nM), which is highly selective toward Mps-1, relative to other protein and lipid kinases.51 CFI-402257 has recently entered a Phase 1 clinical trial that is aimed to study safety, tolerability and pharmacokinetics of the compound in patients with advanced solid cancers (ClinicalTrails.gov Identifier: NCT02792465).
Here, for the first time we targeted chromosomal instability in MM. We found that inhibition of Mps-1 selectively suppressed viability of MM enabling cells to bypass the SAC and causing perturbations of the mitotic process, ultimately leading to cell death. Importantly, CFI-402257 was effective in an orthotopic, syngeneic MM model. We demonstrated that the mitotic kinase Mps-1, so far unrecognized as a contributing factor in the pathology of MM, constitutes a promising therapeutic target. We propose that CFI-402257 fits the criteria for further clinical investigation and could offer a novel therapeutic approach to patients with MM. Based on our in vivo data, the potential clinical application of CFI-402257 would be beneficial in combination with established chemotherapeutic regimens. Adding CFI-402257 to the first-line treatment should benefit those patients who fail to respond to conventional chemotherapy. It is hoped that future biomarker studies may help identify those patients that are more likely to benefit from this therapy.11, 56, 57
Supplementary Material
Acknowledgments
M.C. provides consultation for mesothelioma diagnosis.
This work was supported in part by the NCI-R01 CA198138 to M.C.; by the NCI-R01 CA160715 to H.Y.; and by the University of Hawai’i Foundation, which received an unrestricted gift to support MM research from Honeywell International Inc., to M.C.; and from United-4-a-Cure, to M.C. and H.Y.
CFI-402257 was synthesized and provided by our collaborators and co-authors at the Campbell Family Institute for Breast Cancer Research, Toronto, Canada.
Abbreviations
- Cis/Pem
Cisplatin/Pemetrexed
- EDTA
Ethylenediaminetetraacetic acid
- FBS
Fetal Bovine Serum
- FITC
Fluorescein isothiocyanate
- i.p.
Intraperitoneally
- IVIS
In vivo imaging system
- MM
Malignant mesothelioma
- Mps-1
Monopolar spindle 1
- PARP
Poly(ADP-Ribose) Polymerase
- PI
Propidium Iodide
- PVDF
Polyvinylidenefluoride
- TTK
Dual specificity Thr/Tyr kinase
- TRIS
Trisaminomethane
Footnotes
Conflict of Interest
All other authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- 1.Carbone M, Ly BH, Dodson RF, Pagano I, Morris PT, Dogan UA, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol. 2012;227(1):44–58. doi: 10.1002/jcp.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henley SJ, Larson TC, Wu M, Antao VC, Lewis M, Pinheiro GA, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003–2008. Int J Occup Environ Health. 2013;19(1):1–10. doi: 10.1179/2049396712Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89(10):716–24. 24A–24C. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone M, Kanodia S, Chao A, Miller A, Wali A, Weissman D, et al. Consensus Report of the 2015 Weinman International Conference on Mesothelioma. J Thorac Oncol. 2016;11(8):1246–62. doi: 10.1016/j.jtho.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 6.Isobe H, Wellham L, Sauerteig A, Sridhar KS, Ramachandran C, Krishan A. Doxorubicin retention and chemoresistance in human mesothelioma cell lines. Int J Cancer. 1994;57(4):581–5. doi: 10.1002/ijc.2910570423. [DOI] [PubMed] [Google Scholar]
- 7.Comertpay S, Pastorino S, Tanji M, Mezzapelle R, Strianese O, Napolitano A, et al. Evaluation of clonal origin of malignant mesothelioma. J Transl Med. 2014;12:301. doi: 10.1186/s12967-014-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75(2):264–9. doi: 10.1158/0008-5472.CAN-14-1008. [DOI] [PubMed] [Google Scholar]
- 9.Singhi AD, Krasinskas AM, Choudry HA, Bartlett DL, Pingpank JF, Zeh HJ, et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol. 2016;29(1):14–24. doi: 10.1038/modpathol.2015.121. [DOI] [PubMed] [Google Scholar]
- 10.Lo Iacono M, Monica V, Righi L, Grosso F, Libener R, Vatrano S, et al. Targeted next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: a retrospective study. J Thorac Oncol. 2015;10(3):492–9. doi: 10.1097/JTO.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 11.Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48(4):407–16. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 12.Ugurluer G, Chang K, Gamez ME, Arnett AL, Jayakrishnan R, Miller RC, et al. Genome-based Mutational Analysis by Next Generation Sequencing in Patients with Malignant Pleural and Peritoneal Mesothelioma. Anticancer Res. 2016;36(5):2331–8. [PubMed] [Google Scholar]
- 13.Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol. 2015;10(4):565–76. doi: 10.1097/JTO.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagemeijer A, Versnel MA, Van Drunen E, Moret M, Bouts MJ, van der Kwast TH, et al. Cytogenetic analysis of malignant mesothelioma. Cancer Genet Cytogenet. 1990;47(1):1–28. doi: 10.1016/0165-4608(90)90258-c. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi T, Jhanwar SC, Siegfried JM, Keller SM, Testa JR. Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res. 1993;53(18):4349–55. [PubMed] [Google Scholar]
- 16.Zeiger MA, Gnarra JR, Zbar B, Linehan WM, Pass HI. Loss of heterozygosity on the short arm of chromosome 3 in mesothelioma cell lines and solid tumors. Genes Chromosomes Cancer. 1994;11(1):15–20. doi: 10.1002/gcc.2870110104. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi T, Karnan S, Fukui T, Yokoyama T, Tagawa H, Yokoi K, et al. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci. 2007;98(3):438–46. doi: 10.1111/j.1349-7006.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshikawa Y, Emi M, Hashimoto-Tamaoki T, Ohmuraya M, Sato A, Tsujimura T, et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. Proc Natl Acad Sci U S A. 2016;113(47):13432–7. doi: 10.1073/pnas.1612074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jean D, Thomas E, Manie E, Renier A, de Reynies A, Lecomte C, et al. Syntenic relationships between genomic profiles of fiber-induced murine and human malignant mesothelioma. Am J Pathol. 2011;178(2):881–94. doi: 10.1016/j.ajpath.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napolitano A, Pellegrini L, Dey A, Larson D, Tanji M, Flores EG, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene. 2016;35(15):1996–2002. doi: 10.1038/onc.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72(13):3290–301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Beuschel SL, Butnor KJ, et al. Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Ann N Y Acad Sci. 2010;1203:7–14. doi: 10.1111/j.1749-6632.2010.05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10):1022–5. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone M, Flores EG, Emi M, Johnson TA, Tsunoda T, Behner D, et al. Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s. PLoS Genet. 2015;11(12):e1005633. doi: 10.1371/journal.pgen.1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazdar AF, Carbone M. Molecular pathogenesis of malignant mesothelioma and its relationship to simian virus 40. Clin Lung Cancer. 2003;5(3):177–81. doi: 10.3816/CLC.2003.n.031. [DOI] [PubMed] [Google Scholar]
- 26.Carbone M. Simian virus 40 and human tumors: It is time to study mechanisms. J Cell Biochem. 1999;76(2):189–93. doi: 10.1002/(sici)1097-4644(20000201)76:2<189::aid-jcb3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Bononi A, Yang H, Giorgi C, Patergnani S, Pellegrini L, Su M, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 2017 doi: 10.1038/cdd.2017.95. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bononi A, Giorgi C, Patergnani S, Larson D, Verbruggen K, Tanji M, et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature. 2017 doi: 10.1038/nature22798. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5(10):773–85. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 30.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10(7):478–87. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stucke VM, Sillje HH, Arnaud L, Nigg EA. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 2002;21(7):1723–32. doi: 10.1093/emboj/21.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Winey M. The MPS1 family of protein kinases. Annu Rev Biochem. 2012;81:561–85. doi: 10.1146/annurev-biochem-061611-090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nijenhuis W, von Castelmur E, Littler D, De Marco V, Tromer E, Vleugel M, et al. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J Cell Biol. 2013;201(2):217–31. doi: 10.1083/jcb.201210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Z, Gao H, Yu H. CELL DIVISION CYCLE. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science. 2015;348(6240):1260–4. doi: 10.1126/science.aaa4029. [DOI] [PubMed] [Google Scholar]
- 35.Jelluma N, Brenkman AB, van den Broek NJ, Cruijsen CW, van Osch MH, Lens SM, et al. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132(2):233–46. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 36.Fisk HA, Mattison CP, Winey M. A field guide to the Mps1 family of protein kinases. Cell Cycle. 2004;3(4):439–42. [PubMed] [Google Scholar]
- 37.Fisk HA, Mattison CP, Winey M. Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc Natl Acad Sci U S A. 2003;100(25):14875–80. doi: 10.1073/pnas.2434156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei JH, Chou YF, Ou YH, Yeh YH, Tyan SW, Sun TP, et al. TTK/hMps1 participates in the regulation of DNA damage checkpoint response by phosphorylating CHK2 on threonine 68. J Biol Chem. 2005;280(9):7748–57. doi: 10.1074/jbc.M410152200. [DOI] [PubMed] [Google Scholar]
- 39.Ling Y, Zhang X, Bai Y, Li P, Wei C, Song T, et al. Overexpression of Mps1 in colon cancer cells attenuates the spindle assembly checkpoint and increases aneuploidy. Biochem Biophys Res Commun. 2014;450(4):1690–5. doi: 10.1016/j.bbrc.2014.07.071. [DOI] [PubMed] [Google Scholar]
- 40.Daniel J, Coulter J, Woo JH, Wilsbach K, Gabrielson E. High levels of the Mps1 checkpoint protein are protective of aneuploidy in breast cancer cells. Proc Natl Acad Sci U S A. 2011;108(13):5384–9. doi: 10.1073/pnas.1007645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwiatkowski N, Jelluma N, Filippakopoulos P, Soundararajan M, Manak MS, Kwon M, et al. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat Chem Biol. 2010;6(5):359–68. doi: 10.1038/nchembio.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo R, Caldarelli M, Mennecozzi M, Giorgini ML, Sola F, Cappella P, et al. Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res. 2010;70(24):10255–64. doi: 10.1158/0008-5472.CAN-10-2101. [DOI] [PubMed] [Google Scholar]
- 43.Jemaa M, Galluzzi L, Kepp O, Senovilla L, Brands M, Boemer U, et al. Characterization of novel MPS1 inhibitors with preclinical anticancer activity. Cell Death Differ. 2013;20(11):1532–45. doi: 10.1038/cdd.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tardif KD, Rogers A, Cassiano J, Roth BL, Cimbora DM, McKinnon R, et al. Characterization of the cellular and antitumor effects of MPI-0479605, a small-molecule inhibitor of the mitotic kinase Mps1. Mol Cancer Ther. 2011;10(12):2267–75. doi: 10.1158/1535-7163.MCT-11-0453. [DOI] [PubMed] [Google Scholar]
- 45.Janssen A, Kops GJ, Medema RH. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc Natl Acad Sci U S A. 2009;106(45):19108–13. doi: 10.1073/pnas.0904343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason J, Wei X, Fletcher G, Kiarash R, Brokx R, Hodgson R, et al. Functional Characterization of CFI-402257, a Potent and Selective Mps1/TTK Kinase Inhibitor, for the Treatment of Cancer. Proc Natl Acad Sci U S A. 2017;114(12):3127–32. doi: 10.1073/pnas.1700234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyerhoff RR, Yang CF, Speicher PJ, Gulack BC, Hartwig MG, D'Amico TA, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res. 2015;196(1):23–32. doi: 10.1016/j.jss.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006;103(27):10397–402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274(36):25543–9. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Laufer R, Patel NK, Ng G, Sampson PB, Li SW, et al. Discovery of Pyrazolo[1,5-a]pyrimidine TTK Inhibitors: CFI-402257 is a Potent, Selective, Bioavailable Anticancer Agent. ACS Med Chem Lett. 2016;7(7):671–5. doi: 10.1021/acsmedchemlett.5b00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23(16):2825–37. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 53.Mezzapelle R, Rrapaj E, Gatti E, Ceriotti C, Marchis FD, Preti A, et al. Human malignant mesothelioma is recapitulated in immunocompetent BALB/c mice injected with murine AB cells. Sci Rep. 2016;6:22850. doi: 10.1038/srep22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Jaarsveld RH, Kops GJPL. Difference Makers: Chromosomal Instability versus Aneuploidy in Cancer. Trends in Cancer. 2016;2(10):561–71. doi: 10.1016/j.trecan.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Cohen P. Protein kinases--the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1(4):309–15. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 56.Ostroff RM, Mehan MR, Stewart A, Ayers D, Brody EN, Williams SA, et al. Early detection of malignant pleural mesothelioma in asbestos-exposed individuals with a noninvasive proteomics-based surveillance tool. PLoS One. 2012;7(10):e46091. doi: 10.1371/journal.pone.0046091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Napolitano A, Antoine DJ, Pellegrini L, Baumann F, Pagano I, Pastorino S, et al. HMGB1 and Its Hyperacetylated Isoform are Sensitive and Specific Serum Biomarkers to Detect Asbestos Exposure and to Identify Mesothelioma Patients. Clin Cancer Res. 2016;22(12):3087–96. doi: 10.1158/1078-0432.CCR-15-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao JQ, Li Q, Xu SP, Shen YX, Sun GY. Effect of lumiracoxib on proliferation and apoptosis of human nonsmall cell lung cancer cells in vitro. Chin Med J (Engl) 2008;121(7):602–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.