Figure 1.
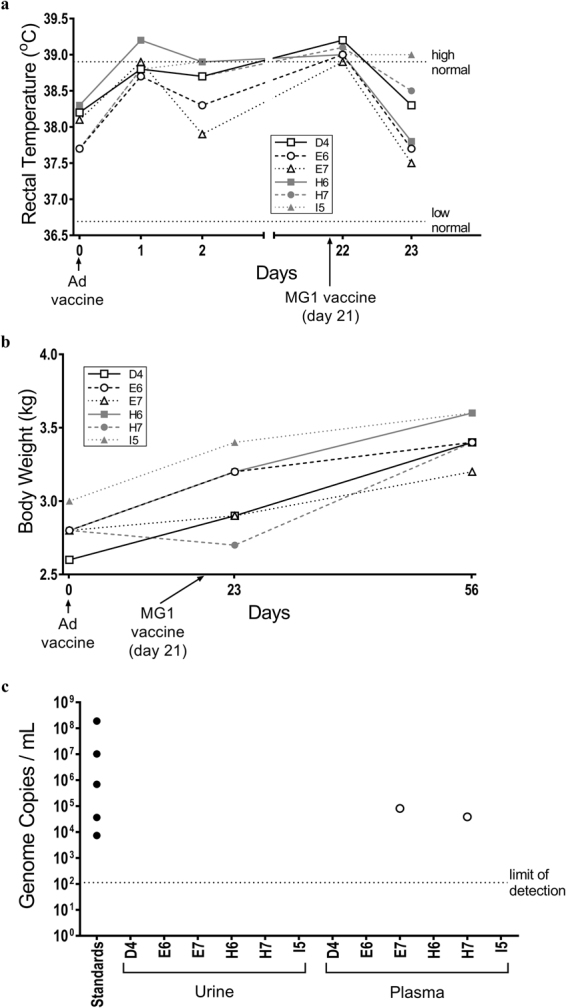
Results of a pilot study to assess the safety of Maraba virus in cats. Six healthy purpose-bred research cats were vaccinated intramuscularly with 1 × 1010 pfu of an E1/E3-deleted replication-deficient recombinant human serotype 5 adenovirus (Ad) expressing the melanoma-associated antigen human dopachrome tautomerase (hDCT) on day 0. On day 21 cats were boosted by intravenous infusion of 2 × 109 (cats #H6 and H7), 2 × 1010 (cats #I5 and D4) or 2 × 1011 (cats #E6 and E7) pfu of a replication-competent Maraba virus (MG1) expressing hDCT. (a) Rectal temperatures and (b) weights were monitored. (c) Urine and plasma samples acquired on day 23 of the study (i.e. two days post-boost) were assessed for the presence of Maraba virus genomes by quantitative RT-PCR. Data values for each individual cat are shown.
