Abstract
Background
Colorectal cancer (CRC) incidence is rising among patients under age 50. As such, we set out to determine the proportion of CRC-related hospital admissions and distribution of colon cancer by stage in different age groups.
Methods
The NIS database for 2002–2012 was used to investigate trends of colorectal cancer resection by age and the ACS-NSQIP database for 2012–2013 was used to investigate contemporary stage at diagnosis for colon cancer in different age groups.
Results
A total of 1,198,421 patients were admitted to a hospital with a diagnosis of CRC, and captured by the NIS database. Although the number of hospitalized CRC patients decreased from 2002–2012, the observed decrease was predominantly in patients older than 65 years (P<0.01) and in colon cancer compared to rectal cancer patients (P<0.01). The proportion of patients younger than 65 years increased from 32.8% in 2002 to 41.1% in 2012, and the proportion of patients under age 50 increased from 9% to 12%. In the NSQIP database, the age <50 group also had a significantly higher proportion of advanced disease (stage III/IV) compared to patients age 50 and older (66% vs. 47%, P<0.01). In 2012 it was observed that most patients with rectal cancer were younger than 65 years (55.8%).
Conclusion
There was a steady decrease in the number of hospitalized patients with colorectal cancer during the last decade, primarily attributable to a decrease in the older than 65 years age patients and colon cancer patients. The proportion of hospitalized patients age <50 is rising. In addition, patients younger than 50 years were more likely to have advanced disease compared to older patients.
Keywords: Colorectal Cancer, Screening
Introduction
Colorectal cancer is one of the most common malignant neoplasms in the United States1–4. The high prevalence and identifiable precursor lesions of colorectal cancer make it ideal for screening 5. The incidence of colorectal cancer has declined during the last decade, which has been attributed to colorectal screening in at risk populations (e.g. older than 50 years)5,6. However, the burden of the disease is disproportionate within demographic subpopulations5,6. The significant decrease in the incidence of colorectal cancer due to screening programs as well as cost effectiveness of colorectal screening have been consistently cited in the literature7–11. The American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) confirmed a decline in incidence and mortality of colorectal cancer during the last decade8. However, recent studies have reported an increase in the incidence of colorectal cancer diagnosis among young adults, in contrast to the decreasing rates observed for adults in the screened population (aged 50 and above) 12–14. This study aimed to report contemporary trends in the different age groups of colorectal cancer patients during the recent decade and compare stage of the disease at diagnosis in young adults and older populations.
Materials and Methods
This study evaluated patients who were hospitalized with the diagnosis of colorectal cancer in the US during 2002–2012. Primary endpoints were trends of colorectal cancer hospitalization by age and contemporary stage for CRC in different age groups. Data were derived from two United States national databases. The Nationwide Inpatient Sample (NIS) database for 2002–2012 was used to investigate trends of colorectal cancer hospitalization by age. The NIS is the largest inpatient health care database in the United States and is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP) 15. It contains de-identified data on nearly eight million hospital stays each year across the United States with an approximately 20% stratified sample of the American community, nonmilitary, and nonfederal hospitals, representing over 97 percent of the US population 15. We included patients admitted to a hospital with the diagnosis of colon or rectal cancer who underwent a colorectal resection. This included either patients who were admitted with colorectal cancer or patients who were admitted with other diagnoses and were diagnosed with colorectal cancer during hospitalization. We included all electively and non-electively admitted patients in the study. However, in order to exclude patients with multiple admissions from the study we only included patients who underwent colorectal resection during hospitalization. Colorectal resection was defined according to ICD-9 codes of: 45.71–45.83, 48.40–48.69, and 1731-17.39 and colorectal cancer patients were diagnosed according to ICD-9 codes of: 153, 154, 153.0–154.9, 230.3, and 230.4 according to patient’s pathology or surgeon’s report. Also, the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for 2012–2013 was used to investigate the contemporary stage of colon cancer in different age groups. Due to limitations of the database, the stage of rectal cancer cases is not available, therefore we only could investigate the stage of colon cancer cases. ACS NSQIP is a nationally validated program that prospectively collects detailed clinical data of surgical patients preoperatively through thirty days after operation in the United States16. Patients with missing data regarding age in the NIS database and patients with missing data regarding cancer stage in the NSQIP database were excluded from the study. Approval for the use of the data in this study was obtained from the Institutional Review Board of the University of California, Irvine Medical Center, NIS, and NSQIP. Patients’ ages were classified into five groups of younger than 40, between 40 and 49, between 50 and 65, between 66 and 79, and more than 79 year. “Young adults” were defined as patients younger than 50 years of age. The population for colorectal screening was defined as patients’ age 50 years and older. Colon cancer stages were classified into four groups of stage I, II, III, and IV according to the 7th edition of the American Joint Committee on Cancer Staging Manual17.
Statistical Analysis
Data analyses were performed using the SPSS® software, Version 22 (SPSS Inc., Chicago, IL). The primary analysis involved multivariate analysis using logistic regression. Multivariate logistic regression was used to compare different groups of patients for binary outcomes. We adjusted for demographic factors (age, sex, and race). The odds ratio (OR) with a 95% confidence interval was calculated for each correlation. The level of significance used for retention was 0.05.
Results
The study population consisted of 1,198,421 patients who were admitted to NIS hospitals with the diagnosis of colorectal cancer in the US from 2002–2012. Of these, 68.9% were electively admitted. Overall, 77% of patients had colon cancer and 23% had rectal cancer. The median age of patients was 70 years old; the majority of patients were Caucasian (77.8%) and female (50.4%). The median ages of patients with rectal and colon cancer were 65 and 71 years old respectively. Rectal cancer patients were significantly younger than colon cancer patients (adjusted mean difference = 5 years, P<0.01). The median ages of White, African American, Hispanic, and Asian patients were 71, 64, 66, and 66 years respectively (P<0.01). The summary of patient characteristics is shown in Table 1.
Table 1.
Demographics of patients admitted to hospitals with diagnosis of colorectal cancer in the US during 2002–2012 (NIS)
| Age Band |
Gender | Admissio n |
Cancer Site | Hospitalization Location |
Race | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Elective | Right Sided Colon Cancer* |
Left Sided Colon Cancer* * |
Rectal Cancer* ** |
Urban | White | African America n |
Hispan ic |
Asian | |
| Total | 594074 (49.6%) | 824161 (68.9%) | 546275 (45.6%) | 304293 (25.4%) | 275303 (23%) | 1040149(87.3%) | 743802 (77.8%) | 96269 (10.1%) | 61242 (6.4%) | 27023 (2.8%) |
| Age<40 | 13384 (50.3%) | 17567 (66.1%) | 8906 (33.4%) | 7660 (28.7%) | 8621 (32.3%) | 24406(92.1%) | 13581 (63.4%) | 2931 (13.7%) | 2909 (13.6%) | 982 (4.6%) |
| 40≤Age< 50 | 43800 (51.8%) | 60701 (71.5%) | 26547 (31.2%) | 24976 (29.3%) | 29378 (34.5%) | 76965(90.9%) | 46509 (69.2%) | 9537 (14.2%) | 6099 (9.1%) | 2646 (3.9%) |
| 50≤Age ≤65 | 197175 (54.5%) | 264835 (73.4%) | 132215 (36.5%) | 102072 (28.2%) | 106407 (29.4%) | 318760(88.7%) | 210516 (73%) | 38227 (13.2%) | 21001 (7.3%) | 9344 (3.2%) |
| 65< Age <80 | 232657 (51.2%) | 322966 (71.2%) | 218428 (48%) | 113295 (24.9%) | 93609 (20.6%) | 389807(86.2%) | 287916 (79.5%) | 33014 (9.1%) | 22009 (6.1%) | 9521 (2.6%) |
| Age ≥80 | 107058 (39.6%) | 158092 (58.7%) | 160179 (59.3%) | 56290 (20.8%) | 37288 (13.8%) | 230219(85.7%) | 185280 (85.7%) | 12560 (5.8%) | 9224 (4.3%) | 4530 (2.1%) |
Include cecum, ascending colon, hepatic flexure, and transverse colon
Include splenic flexure, descending colon, sigmoid
Includes recto-sigmoid junction tumors
There was a steady decrease in the annual number of patients who were admitted to a hospital with a diagnosis of colorectal cancer and underwent colorectal resection from 117,754 patients in 2002 to 98,175 patients in 2012. The decrease was seen only for colon cancer operations (93,588 vs. 72,300, P<0.01) and the annual hospitalization number of rectal cancer resection was increased (24,166 vs. 25,875). Also, the proportion of non-electively admitted patients significantly decreased during the period of the study (33.5% vs. 28.7%, P<0.01). In addition, we found a dramatic decrease in the number of hospitalized colorectal cancer patients during the period of 2008 to 2009 (111121vs. 107464).
The proportion of patients younger than 40, age 40–49, and age 50–65 years increased during the 10 years of the study period (Figure 1). However, the proportion of patients older than 65 years decreased from 67.2% in 2002 to 58.9% in 2012. A concomitant increase in proportion of patients younger than age 65 was seen in both colon cancer and rectal cancer patients (P<0.01) during the study period (Figure 2 and 3). The increase in the proportion of patients younger than 65 years was significantly higher in rectal cancer patients compared to colon cancer patients (P<0.01) (Figure 4). As a result, by 2007 >50% of rectal cancer patients were age <65, and in 2012 patients age <65 represented 55.8% of all rectal cancer patients. However, only 35.8% of patients with colon cancer were younger than age 65 in 2012.
Figure 1.

Trends in the Relative Weighted Numbers of cases in each Age Group among Rectal and Colon Cancers, NIS 2002–2012. Year 2002 equals 100.
Figure 2.
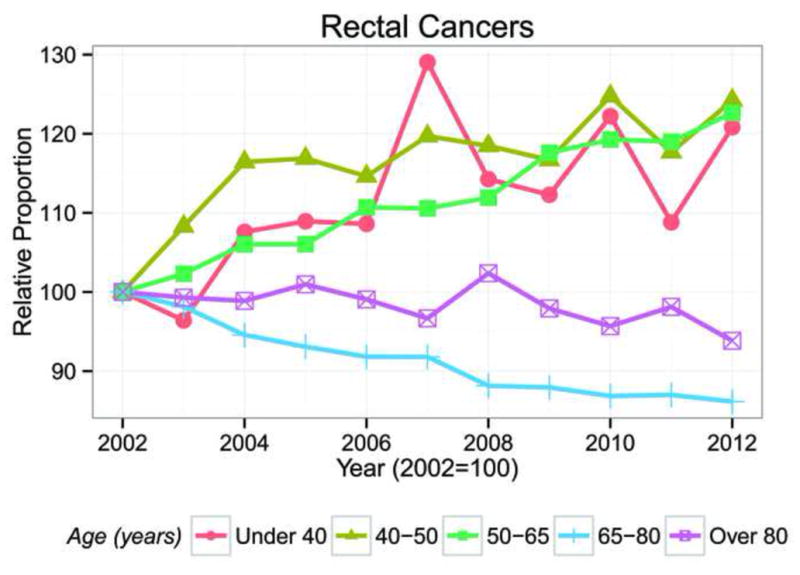
Trends in the Numbers of rectal cancer patients in each Age Group, NIS 2002–2012. Year 2002 equals 100.
Figure 3.

Trends in the Numbers of colon cancer patients in each Age Group, NIS 2002–2012. Year 2002 equals 100.
Figure 4.

Proportion of patients younger than 65 years in colon and rectal cancer over time
Right colon (45.6%) was the most common site of colorectal cancer followed by the left colon (25.4%) and rectum (23%). However, the frequency of rectal cancer was significantly higher in patients younger than age 50 compared to older patients (34% vs. 21.6%, AOR: 1.86, CI: 1.84–1.89, P<0.01). There was a small decrease in the frequency of left sided colon cancers (27.6% vs. 23.1%) and right sided colon cancer (46.2% vs. 43.8%) during the study period. However, the frequency of rectal cancer increased during the same time period (20.5% vs. 26.4%).
The number of hospitalizations for colorectal cancer has decreased in both urban and rural hospitals (99545 vs. 87105 and 17816 vs10740, respectively, P<0.01). The trends of hospitalization for colorectal cancer in rural hospitals decreased during 11 years of study from 15.2% in 2002 to 11% in 2012 (P<0.01). Also, hospitalization for colorectal cancer for patients older than 65 years decreased in both urban (66.5% vs. 58%, P<0.01) and rural hospitals (71% vs. 66.2%, P<0.01). However, hospitalization for colorectal cancer for patients older than 65 years was higher in rural hospitals compared to urban hospitals (69.3 vs. 62%, P<0.01).
The stage of colon cancer at the time of operation by age is reported in Table 2 according to the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Overall, 61% of patients younger than 50 years had stage III or IV colon cancer, while only 47.5% of patients 50 years and older had stage III or IV diseases. Following adjustment for sex and race, young adults had significantly higher risk of advanced disease (AOR: 1.73, CI: 1.48–2.02, P<0.01).
Table 2.
Stage of colon cancer at diagnosis by age (NSQIP 2012–2013)
| Age Band | Stage of Colon Cancer | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Age<40 | 27 (14%) | 29 (15%) | 81 (42%) | 56 (29%) |
| 40≤Age<50 | 80 (15.9%) | 126 (25%) | 170 (33.8%) | 127 (25.2%) |
| 50≤Age ≤65 | 422 (21%) | 550 (27.3%) | 646 (32.1%) | 394 (19.6%) |
| 65≤ Age <80 | 491 (24.3%) | 593 (29.3%) | 623 (30.9%) | 314 (15.5%) |
| Age ≥80 | 211 (20%) | 404 (38.2%) | 312 (29.5%) | 130 (12.3%) |
| Total | 1231 (21.3%) | 1702 (29.4%) | 1835 (31.7%) | 1021 (17.6%) |
Discussion
In this study, we observed a steady decrease in the number of patients who had surgery for a diagnosis of colorectal cancer from 2002 through 2012. This is consistent with prior reports of declining CRC incidence rates, estimated at 2.3%–2.6% annually starting from 19998,18–20. Surprisingly, we found a dramatic decrease in the number of hospitalized colorectal cancer patients in the period of 2008 to 2009. Although we cannot give any definitive explanation for this observation, it may be explained by the increase in uptake of colorectal screening in the US by the recommendation of US Preventive Services Task Force (USPSTF) in 200821. The USPSTF recommended screening for colorectal cancer using fecal occult blood testing, sigmoidoscopy, or colonoscopy in adults, beginning at age 50 years and continuing until age 75 years 21. When evaluating the trends of the decrease in colorectal cancer hospitalization, such trends exist for colon cancer but not rectal cancer. Also, the number of colorectal cancer patients in the over 65 year age group decreased during the last decade, while the number of CRC patients under age 65 remained relatively stable. Drawing a conclusion without detailed information of changes in the US population in each age group is difficult, although the overall aging of the US population is well described. Therefore, the decrease in hospitalization seen mostly in the population older than 65 years is likely clinically relevant. Similar results have been reported previously, including the observed decrease in colon cancer admissions compared to rectal cancer13. Further studies are indicated to explain these trends.
Our study analysis shows a significant decrease in the number of admitted patients who were above the age of recommended colorectal screening (age 50 and older) during the last decade. This is most likely attributable to increased utilization of screening, especially colonoscopy, during the last decade. Similar trends were reported previously13. The Centers for Disease Control and Prevention (CDC) reported a frequency of 65.4% for colorectal screening for the at risk population in the US in 201222. However, we found the age group of 50–64 years had essentially no or minimal decrease in admissions, with the over 65 year age group accounting for the change. A poor adoption of colorectal screening in the population aged 50–64 years compared to those aged 65–75 years in the US has been reported previously (55% vs. 68%)23. Barriers to colon cancer screening in the population of 50–64 years of age needs more investigation.
Surprisingly, in 2012 a point was reached where most patients with rectal cancer were younger than 65 years (55.8%). The increase in the rate of rectal cancer in young adults during the last decade has been reported previously, particularly in certain race/ethnic groups 12,13. Possible reasons for the increase in the rate of colorectal cancer in young adults are the increased prevalence of obesity and type II diabetes over the last three decades, which are risk factors for colorectal cancer13,18,24,25. In addition, a recently published article reported worse molecular features of early onset colorectal adenocarcinoma compared to tumors in older populations26. Considering the increase in the number of hospitalized rectal cancer patients was predominantly in the age group of 50–64 years in our study, further studies are indicated to evaluate how to improve rates of colorectal screening for this age group in the US. Additionally, since prevention requires colon cancer screening prior to the age of onset, to impact this age group, screening may need to be done earlier.
Our analysis shows young adult patients (younger than 50) who were hospitalized with colon cancer have more advanced disease compared to older patients. We found 61% of young adult patients with colon cancer had stage III or IV disease during 2012–2013. However, only 47.5% of older patients had such advanced disease (P<0.01). The difference is likely related to screening of colorectal cancer in older patients, which results in diagnosis of the disease at earlier stages. We found the highest frequency of metastatic disease in patients younger than 40 years. The higher rate of advanced colon cancer in young adults has been reported previously12,27–31 and is of particular concern since advanced disease is often not curable. Cost benefits of colorectal screening in population younger than 50 needs more investigation and consideration.
We have observed that rectal cancer is more common among the younger patient population compared to colon cancer. This is in line with previous reports of a higher proportion of left sided colorectal cancer compared to right sided colorectal cancers in young adults.26,32 Chang et al. reported colorectal adenocarcinoma in young adults has a striking predilection for the distal colon, particularly the sigmoid colon and rectum26. In addition we found the population of colorectal cancer patients is getting younger over time. Similar trends have been cited multiple times 12,13,28,29. Surprisingly, we found this trend is more pronounced in rectal cancer compared to colon cancer. These findings require further investigation.
Our study results show the rate of hospitalization for colorectal cancer in patients younger than 65 years is higher in urban hospitals compared to rural hospitals. However, 87.3% of hospitalization episodes for colorectal cancer were in urban hospitals and it is difficult to draw any conclusion regarding distribution of age in colorectal cancer according to rural and urban hospitals. Further investigations are needed regarding trends of colorectal cancer incidence in rural and urban regions. Also, we found 77.8% of patients who were hospitalized for colorectal cancer were Caucasian. Given limitations in our databases we cannot compare the relative risk for colorectal cancer in Caucasian adults with African American adults. A higher rate of colorectal cancer in African Americans compared to Caucasian patients was previously reported 8. We found Caucasian patients with colorectal cancer to be significantly older than the other races, which is consistent with the significant increase in the rate of colorectal cancer in young African American adults which has been reported during the last decade 13. This may be related to a higher risk of colorectal cancer in African American adults or lower access for colorectal screening in African Americans 23,33. Further efforts in educational programs for colorectal screening are needed 33.
Study Limitations
This study is a retrospective review and is subject to typical biases for retrospective studies such as selection bias and coding inaccuracies. Some patients might have multiple admissions and we could not identify such patients. The NIS database only provides information about patients with colorectal cancer during hospitalization and the NSQIP database provides information on patients with colorectal cancer up to 30 days after operation and therefore we do not have any information on long term outcomes of patients. The NSQIP database does not provide any clinical information on the stage of the disease for rectal cancer patients. Also the information of the cancer stage for patients with colon cancer is only provided for 2012–2013, so we could not investigate trends of the colon cancer stage by age over time. Demographic factors were used for adjustment in the analysis; however, some unmeasured confounding variables may exist. Despite these limitations, the present analysis can be used as a baseline in future strategies and studies of prevention of colorectal cancer.
Conclusion
There was a steady increase in the number of patients younger than 65 years who underwent surgery for a diagnosis of colorectal cancer during the last decade in the US. The increase is mostly in 50–64 year old patients who should be considered for colorectal screening. Barriers to colon cancer screening in the population 50–64 years of age needs more investigation and educational programs for improvement in colorectal cancer screening for this population need to be designed. Also, the proportion of admitted young adult patients (younger than 50), who typically are not considered for screening, with colorectal cancer increased from 2002 to 2012. These young adult patients (younger than 50) were also more likely to be diagnosed with advanced stages of colorectal cancer compared to older patients, as we found the highest rate of metastatic disease in patients age 40–49 years. In 2012 we reached a point where the majority of patients with rectal cancer were younger than 65 years of age (55.8%). Future studies to define high risk young adult populations for colorectal cancer are needed as some young adult populations may benefit from earlier colorectal screening.
Footnotes
Abstract was published in the Proceedings of the American Society of Clinical Oncology (ASCO) annual meeting, May 29– Jun 2, 2015, Chicago, Illinois, USA
Disclosures:
Dr. Stamos has received educational grants and speaker fees paid to the Department of Surgery, University of California, Irvine, from Ethicon, Gore, Covidien, and Olympus. Dr. Mills and Dr. Carmichael received Ethicon educational grants paid to the Department of Surgery, University of California, Irvine. Dr. Pigazzi is a consultant for Intuitive Surgical and has also received consultancy fees and educational grants paid to the Department of Surgery, University of California, Irvine. Dr. Moghadamyeghaneh, Dr. Phelan, Dr. Fazl Alizadeh, and Dr. Zen have no disclosures. Dr. Moghadamyeghaneh and Dr. Phelan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest and Source of Funding: Nothing to Disclose
References
- 1.Shike M, Winawer SJ, Greenwald PH, Bloch A, Hill MJ, Swaroop SV. Primary prevention of colorectal cancer. The WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1990;68(3):377–385. [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2014. Atlanta, Ga: American Cancer Society; 2014. [Last accessed May 21, 2014]. Available online. [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005 (based on November 2007 SEER data submission) Bethesda, MD: National Cancer Institute; 2008. Available at: http://seer.cancer.gov/csr/1975_2005/. Updated 2008. [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 5.Inadomi JM. Taishotoyama Symposium Barriers to colorectal cancer screening: economics, capacity and adherence. J Gastroenterol Hepatol. 2008;23(Suppl 2):S198–204. doi: 10.1111/j.1440-1746.2008.05556.x. [DOI] [PubMed] [Google Scholar]
- 6.Jackson-Thompson J, Ahmed F, German RR, Lai SM, Friedman C. Descriptive epidemiology of colorectal cancer in the United States, 1998–2001. Cancer. 2006;107(5 Suppl):1103–1111. doi: 10.1002/cncr.22007. [DOI] [PubMed] [Google Scholar]
- 7.Myer PA, Mannalithara A, Singh G, Ladabaum U. Proximal and distal colorectal cancer resection rates in the United States since widespread screening by colonoscopy. Gastroenterology. 2012;143(5):1227–1236. doi: 10.1053/j.gastro.2012.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolin TD. Cost benefit of early diagnosis of colorectal cancer. Scand J Gastroenterol Suppl. 1996;220:142–146. doi: 10.3109/00365529609094767. [DOI] [PubMed] [Google Scholar]
- 10.Khandker RK, Dulski JD, Kilpatrick JB, Ellis RP, Mitchell JB, Baine WB. A decision model and cost-effectiveness analysis of colorectal cancer screening and surveillance guidelines for average-risk adults. Int J Technol Assess Health Care. 2000;16(3):799–810. doi: 10.1017/s0266462300102077. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan WM, Le Petit C, Berthelot JM, White KJ, Coombs BA, Jones-McLean E. Potential impact of population-based colorectal cancer screening in Canada. Chronic Dis Can. 2003;24(4):81–88. [PubMed] [Google Scholar]
- 12.Singh KE, Taylor TH, Pan CG, Stamos MJ, Zell JA. Colorectal Cancer Incidence Among Young Adults in California. J Adolesc Young Adult Oncol. 2014;3(4):176–184. doi: 10.1089/jayao.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin H, Henley SJ, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014;25(2):191–201. doi: 10.1007/s10552-013-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150(1):17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2000–2010. www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 16.National Surgical Quality Improvement Program [home page on the Internet] Chicago, IL: American College of Surgeons; 2005. [cited 2012 Jan 17]. Available from: www.acsnsqip.org. [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. pp. 113–123. [Google Scholar]
- 18.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992–2008. Cancer Epidemiol Biomarkers Prev. 2012;21(3):411–416. doi: 10.1158/1055-9965.EPI-11-1020. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69(10):866–872. [PubMed] [Google Scholar]
- 20.Schootman M, Lian M, Deshpande AD, McQueen A, Pruitt SL, Jeffe DB. Temporal trends in geographic disparities in small-area-level colorectal cancer incidence and mortality in the United States. Cancer Causes Control. 2011;22(8):1173–1181. doi: 10.1007/s10552-011-9796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Preventive Services Task Force. Screening for colorectal cancer. Recommendation statement. 2008 Oct; www.uspreventiveservicestaskforce.org.
- 22.(CDC) CfDCaP. Vital signs: Colorectal cancer screening, incidence, and mortality--United States, 2002–2010. MMWR Morb Mortal Wkly Rep. 2011;60(26):884–889. [PubMed] [Google Scholar]
- 23.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106(4):dju032. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 25.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 26.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25(8):1128–1139. doi: 10.1038/modpathol.2012.61. [DOI] [PubMed] [Google Scholar]
- 27.Keswani SG, Boyle MJ, Maxwell JP, et al. Colorectal cancer in patients younger than 40 years of age. Am Surg. 2002;68(10):871–876. [PubMed] [Google Scholar]
- 28.Amini AQ, Samo KA, Memon AS. Colorectal cancer in younger population: our experience. J Pak Med Assoc. 2013;63(10):1275–1277. [PubMed] [Google Scholar]
- 29.Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216–224. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 30.O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Ko CY. Are survival rates different for young and older patients with rectal cancer? Dis Colon Rectum. 2004;47(12):2064–2069. doi: 10.1007/s10350-004-0738-1. [DOI] [PubMed] [Google Scholar]
- 31.McKay A, Donaleshen J, Helewa RM, et al. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol. 2014;12:370. doi: 10.1186/1477-7819-12-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahrudin MD, Noori SM. Cancer of the colon and rectum in the first three decades of life. Hepatogastroenterology. 1997;44(14):441–444. [PubMed] [Google Scholar]
- 33.Wu TY, Kao JY, Hsieh HF, et al. Effective colorectal cancer education for Asian Americans: a Michigan program. J Cancer Educ. 2010;25(2):146–152. doi: 10.1007/s13187-009-0009-x. [DOI] [PubMed] [Google Scholar]


