Figure 3.
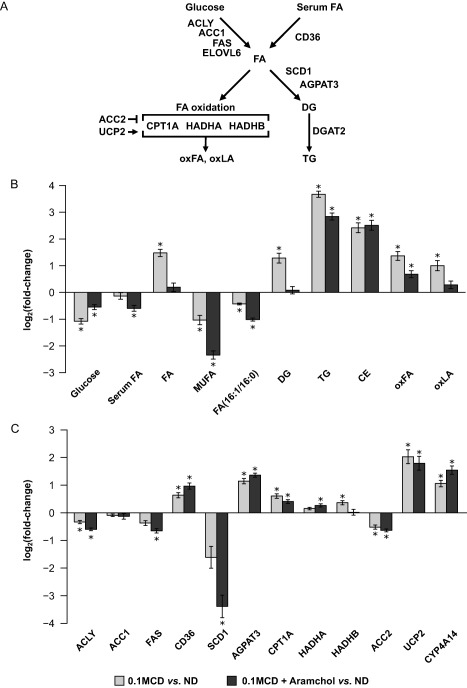
Hepatic lipid metabolism in 0.1MCD‐fed mice treated with vehicle or with Aramchol. (A) Schematic representation of hepatic lipid metabolism. Hepatic FAs originate from serum and through de novo lipogenesis. FAs can either be oxidized in the mitochondria or esterified to form TGs. The rate‐limiting step in mitochondrial β‐oxidation is CPT1A. The accumulation of FAs in the cytoplasm increases their oxidation and generates ROS, which induce GSH depletion and produce oxFA, such as oxLA, which can lead to fibrosis and cell death. (B) Relative fold change (log2) in the hepatic content of the main metabolites involved in lipid metabolism in mice fed a 0.1MCD diet and treated with vehicle or with Aramchol compared to mice fed a normal diet. A 0.1MCD diet led to reduced levels of glucose, serum FAs, and MUFAs and to a reduction in the FA(16:1/16:0) ratio. In contrast, feeding the mice a 0.1MCD diet led to increased levels of FAs, DGs, TGs, CEs, oxFA, and oxLA. (C) Relative fold change (log2) in the content of proteins involved in liver lipid metabolism in mice fed a 0.1MCD diet and treated with vehicle or with Aramchol compared to mice fed a normal diet. Feeding the mice a 0.1MCD diet resulted in reduced levels of ACLY, ACC1, ACC2, FAS, and SCD1. The 0.1MCD mice also showed increased levels of CD36, AGPAT3, CPT1A, HADHA, HADHB, UCP2, and CYP4A14. *P < 0.05. Abbreviations: ACLY, adenosine triphosphate citrate lyase; AGPAT3, 1‐acylglycerol‐3‐phosphate O‐acyltransferase 3; CYP4A14, cytochrome P450, family 4, subfamily a, polypeptide 14; FAS, fatty acid synthase; HADHA, HADHB, 3‐hydroxyacyl‐CoA dehydrogenase A and B; LA, linoleic acid; oxFA, oxidized FA; ND, normal diet; UCP2, uncoupling protein 2. Data were represented as mean ± SEM.
