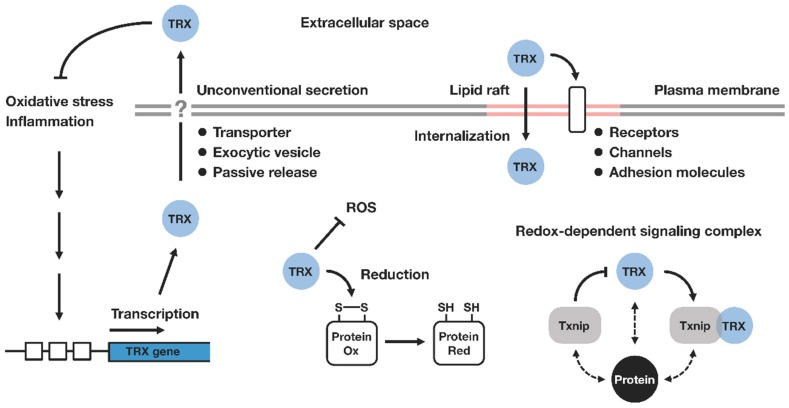
Figure 1.
Thioredoxin-mediated redox regulation. Thioredoxin (TRX) is transcriptionally upregulated in response to environmental or pathological factors associated with oxidative stress. TRX catalyzes the cleavage of protein disulfide bonds, thereby contributing to the maintenance of cellular redox homeostasis. TRX is secreted into the extracellular compartment via ER/Golgi-independent mechanisms (unconventional or nonclassical secretion), where it exhibits its protective effects against inflammation. Extracellular TRX is associated with membrane lipid rafts, where it can control the redox state of cell surface molecules, and influence the downstream signaling pathway. The internalization of extracellular TRX is also mediated through lipid rafts. Intracellularly, TRX interacts with a number of signaling molecules in a redox-dependent fashion. Txnip (also known as TBP-2 or VDUP1) has been identified as a negative regulator of TRX and the binding of Txnip inhibits the reducing activity of TRX. TRX and Txnip form a redox-sensitive signaling complex termed ‘redoxisome’, which may play a central role in the regulation of diverse biological processes ranging from metabolic and immunological pathways to inflammatory response and tumorigenesis. Ox, oxidized; Red, reduced; ROS, reactive oxygen species; TRX, thioredoxin; Txnip, thioredoxin interacting protein.