Fig. 2.
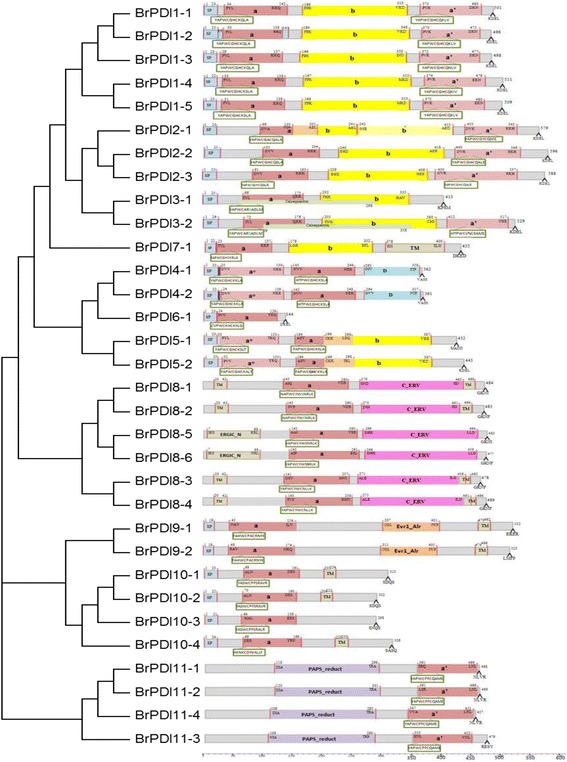
Domain structure of the deduced amino acid sequences of B. rapa PDI genes. The putative signal peptides (SP), the a and b type domains, the N-terminal calcium binding domain calsequestrin, the D domains (Erp29), the transmembrane domains (TM) and the C_ERV (COPII-coatedERV) domain are shown. The thioredoxin-like catalytic domains with two active sites (shown in detail in the boxes) are also shown. Numbers above indicate domain boundaries (aa), and numbers on the right indicate ORF (aa). Domains ao (light gray) and a’ (gray) are homologous to TRX and contain the catalytic CxxC motif (red). Domains b (yellow) and b’ (light yellow) also exhibit a TRX fold, but they do not share high sequence similarity with each other or with domains a or a’. The C-terminal extension (red) contains a (K/H)DEL retention signal for the ER
