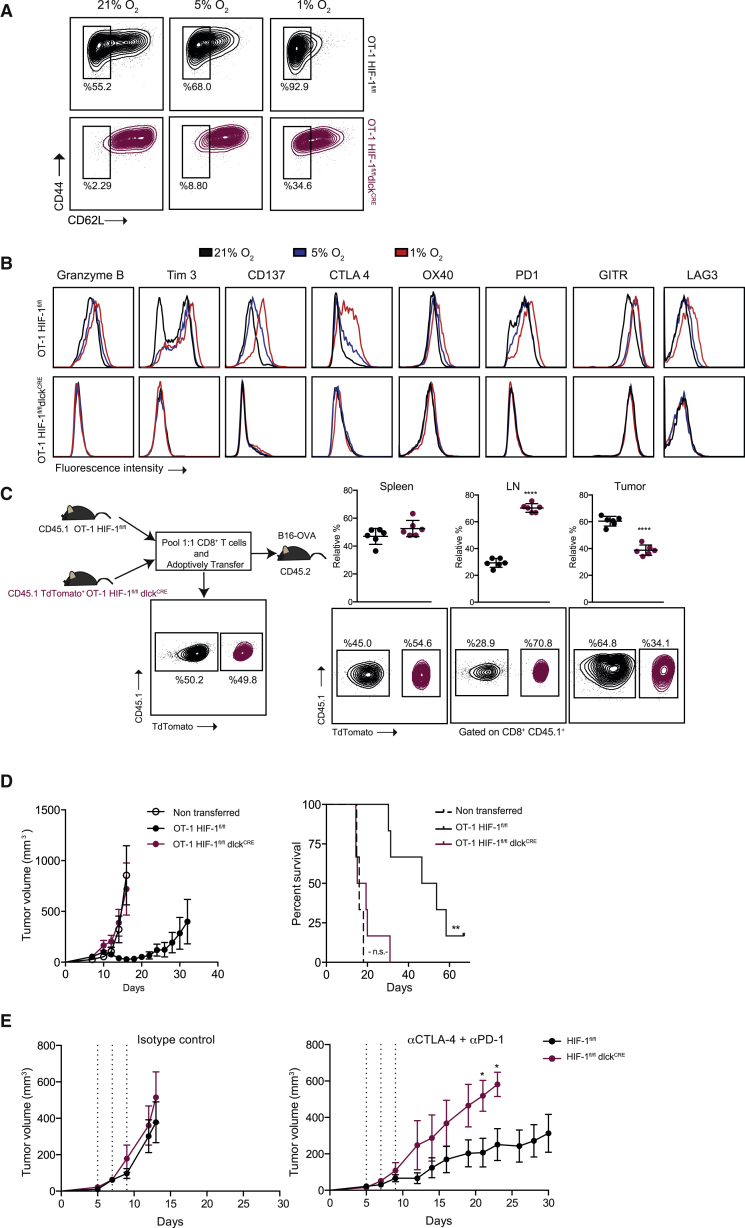
Figure 4.
HIF-1α Is Necessary for Effector CD8+ T Cell Function and Migration
(A) CD8+ T cells were isolated from spleens of OT-1 HIF-1αfl/fldlckCRE (maroon) and littermate control (black) mice and activated with cognate peptide for 2 days, then expanded for 5 days in the presence of IL-2 and subjected to 21%, 5%, or 1% O2 for 24 hr. Representative flow-cytometry histograms showing the expression of CD44 and CD62L (n = 3).
(B) Expression of intracellular granzyme B and the indicated costimulatory molecules/checkpoint receptors on CD8+ T cells prepared as in (A).
(C) Left: diagram outlining the in vivo migration experiment: representative flow-cytometry plots are shown for each pool before and after adoptive cotransfer of HIF-1α mutant (CD45.1+/TdTomato+, maroon) and control (CD45.1+, black) OT-1 T cells into CD45.2+ B16-OVA tumor-bearing WT mice. Right: spleens, lymph nodes (LN), and tumors were collected 48 hr after the adoptive cell cotransfer and relative percentages of migrated cells of the indicated genotypes are shown (error bars represent SD, n = 6).
(D) 1 × 106 OT-1 cells were transferred into recipient mice harboring B16-OVA tumors (n = 6): tumor volumes (left) and overall percent survival (right) are shown; statistical analysis by log-rank (Mantel-Cox) test; error bars represent SEM.
(E) Tumor growth curves of MC38 tumor cells subcutaneously injected into HIF-1α mutant and littermate controls. Mice were treated with either isotype control antibodies (left, n = 3) or a combination of αPD-1 and αCTLA4 blocking antibodies (right, n = 6), on days 5, 7, and 9 (dashed lines). Statistical analysis was performed by two-way ANOVA with Sidak correction for multiple comparisons. Tumor volumes are shown (mean values ± SEM).
∗∗∗∗p < 0.00005, ∗∗p < 0.005, ∗p < 0.05; n.s., not significant. See also Figure S3.