Abstract
Background
Retinitis pigmentosa is a group of inherited disorders characterized by the degeneration of the photoreceptors in the retina, resulting in progressive vision loss. The Argus II system is designed to restore partial functional vision in patients with profound vision loss from advanced retinitis pigmentosa. At present, it is the only treatment option approved by Health Canada for this patient population. In June 2016, Health Quality Ontario published a health technology assessment of the Argus II retinal prosthesis system for patients with advanced retinitis pigmentosa. Based on that assessment, the Ontario Health Technology Advisory Committee recommended against publicly funding the Argus II system for this population. It also recommended that Health Quality Ontario re-evaluate the evidence in 1 year. The objective of this report was to examine new evidence published since the 2016 health technology assessment.
Methods
We completed a health technology assessment, which included an evaluation of clinical benefits and harms, value for money, and patient preferences related to the Argus II system. We performed a systematic literature search for studies published since the 2016 Argus II health technology assessment. We developed a Markov decision-analytic model to assess the cost-effectiveness of the Argus II system compared with standard care, and we calculated incremental cost-effectiveness ratios over a 20-year time horizon. We also conducted a five-year budget impact analysis. Finally, we interviewed people with retinitis pigmentosa about their lived experience with vision loss, and with the Argus II system.
Results
Four publications from one multicentre international study were included in the clinical review. Patients showed significant improvements in visual function and functional outcomes with the Argus II system, and these outcomes were sustained up to a 5-year follow-up (moderate quality of evidence). The safety profile was generally acceptable.
In the base case economic analysis, the Argus II system was cost-effective compared with standard care if the willingness to pay was more than $97,429 per quality-adjusted life-year. We estimated that funding the Argus II system would cost the province $0.71 to $0.78 million per year over 5 years, assuming 4 implants per year.
People with lived experience spoke about the challenges of retinitis pigmentosa, including the gradual but persistent progression of the disease; its impact on their quality of life and their families; and the accessibility challenges they faced. Those who used the Argus II system spoke about its positive impact on their quality of life.
Conclusions
Based on evidence of moderate quality, the Argus II retinal prosthesis system improved visual function, real-life functional outcomes, and quality of life in patients with advanced retinitis pigmentosa. The Argus II system is expensive, but the cost to publicly fund it would be low, because of the small number of eligible patients. The Argus II system can only enable perception of light/dark and shapes/objects, but these advancements represent important gains for people with retinitis pigmentosa in terms of mobility and quality of life.
OBJECTIVE
Health Quality Ontario published a health technology assessment of the Argus II retinal prosthesis system in June 2016.1 Based on that assessment, the Ontario Health Technology Advisory Committee recommended against publicly funding the system. However, the committee also recommended that Health Quality Ontario re-evaluate the evidence in 1 year, because new evidence about the effectiveness of the system was emerging.2
This health technology assessment update looked at new evidence on the clinical benefits and harms, cost-effectiveness, and patient experiences of the Argus II system in patients with bare to no light perception from advanced retinitis pigmentosa to determine whether it should be publicly funded.
BACKGROUND
Health Condition
Retinitis pigmentosa is a group of inherited disorders that involve degeneration of the photoreceptors in the retina. Photoreceptors are the cells that convert light into signals to the brain. People with retinitis pigmentosa gradually lose their vision, eventually progressing to blindness, but the cells in their inner retina are largely preserved.3 This progressive loss of vision creates serious challenges for people with retinitis pigmentosa, affecting their education, employment, mobility, socialization, and mental health, and lowering their quality of life.4
Clinical Need and Target Population
The prevalence of retinitis pigmentosa is 0.04%.5 Based on an estimate from the Foundation Fighting Blindness, approximately 4,000 people in Ontario have some form of retinitis pigmentosa.6
Current Treatment Options
The Argus II retinal prosthesis system is an implantable device designed to restore partial functional vision in patients with bare to no light perception as a result of advanced retinitis pigmentosa. At present, there are no other treatment options.
Health Technology Under Review
The Argus II retinal prosthesis system consists of the following: a 60-electrode implantable array; a video camera mounted in a set of eyeglasses; and an external video-processing unit. The video-processing unit translates visual images captured by the video camera into electrical signals, and then it transmits these signals to the implant. The implant emits small pulses of electricity, bypassing the damaged photoreceptors and stimulating the inner retina cells directly. The visual information from the stimulated retinal cells then travels through the optic nerve to the brain, where the signals are perceived as light patterns in the visual cortex.7
Regulatory Information
The Argus II retinal prosthesis system (Second Sight Medical Products, Inc., Sylmar, California) is licensed by Health Canada as a class III device (licence number 94430).
Ontario Context
The Argus II retinal prosthesis system is not publicly funded in Ontario or in any other Canadian provinces. The University Health Network in Toronto, Ontario, and Maisonneuve-Rosemont Hospital in Montreal, Quebec are the only two centres in Canada that have experience implanting the Argus II system.
The 2016 Argus II health technology assessment described the original selection criteria1 used by the University Health Network for the Argus II system. Since then, based on the centre's experience and the published literature,38 the University Health Network has revised the selection criteria to optimize outcomes in patients receiving the Argus II implant. Now, patients are eligible for the Argus II implant if they meet all of the following criteria:
Blindness with severe to profound retinitis pigmentosa
Bare light perception or no light perception in both eyes. If the patient has no residual light perception, then evidence of intact inner retinal function must be confirmed
Age 45 years or older
Previous history of useful vision
Suitable for surgery and can benefit from the Argus II device based on the University Health Network medical, surgical, and functional assessments to determine residual vision, psychological profile, and functional limitations
Willing to provide informed consent to receive the Argus II implant
Able to complete all follow-up visits at the University Health Network, and has social support to attend visits
Able to follow post-surgical routine and complete the low-vision rehabilitation program
As of May 2017, nine patients (eight from Ontario and one from Saskatchewan) had been implanted with the Argus II retinal prosthesis system at the University Health Network.
Since the publication of the 2016 health technology assessment1 and the related Ontario Health Technology Advisory Committee recommendation,2 the Argus II system has received the following funding and reimbursement approvals:
In November 2016, the United States Centers for Medicare and Medicaid Services finalized its reimbursement of the payment for the surgical procedure and the cost of the Argus II device through codes C1841, C1842, and 0100T9
Effective January 1, 2017, the American Medical Association approved two new category III Current Procedural Terminology codes for the reporting and billing of all services related to implantation and programming of the Argus II system (0472T, 0473T)10
The United Kingdom National Health Service approved funding for 10 patients to receive the Argus II implant in 2017 and planned to collect data to assess how the Argus II system helped patients perform everyday tasks11
The German Institute for the Hospital Remuneration System renewed full approval for the epiretinal prosthesis, allowing hospitals covered under the program to negotiate for reimbursement coverage12
CLINICAL EVIDENCE
Research Question
What are the clinical benefits and harms of the Argus II retinal prosthesis system when used to treat patients with bare to no light perception vision as a result of advanced retinitis pigmentosa?
Methods
We develop research questions in consultation with patients, health care providers, clinical experts, and other health system stakeholders. This re-evaluation of the Argus II retinal prosthesis system searched for and reported on new evidence available since the publication of the 2016 health technology assessment.1
Literature Search
We performed a literature search on February 9, 2017, to retrieve studies published from January 1, 2015, to February 9, 2017. We used the Ovid interface to search the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, National Health Service Economic Evaluation Database (NHSEED), and Database of Abstracts of Reviews of Effects (DARE).
Search strategies were developed by medical librarians using controlled vocabulary (i.e., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.13 Database auto-alerts were created in MEDLINE and Embase and monitored for the duration of the health technology assessment review.
We performed targeted grey literature searching of health technology assessment agency sites and clinical trial registries. We also reviewed reference lists of included studies for any additional studies not identified through the systematic search. See Appendix 1 for literature search strategies, including all search terms.
Literature Screening
A single reviewer reviewed the abstracts and, for those studies meeting the eligibility criteria, we obtained full-text articles. We also examined reference lists for any additional relevant studies not identified through the search.
Types of Studies
We looked at health technology assessments, systematic reviews, meta-analyses, randomized controlled trials, observational studies, and case series that examined the effect of the Argus II retinal prosthesis system in patients with advanced retinitis pigmentosa.
We did not include editorials, abstracts, commentaries, or non-systematic reviews.
Types of Participants
The population of interest was patients receiving the Argus II retinal prosthesis system for advanced retinitis pigmentosa.
Types of Interventions
The intervention of interest was the Argus II retinal prosthesis system.
Types of Outcomes Measures
Visual function
Functional outcomes
Quality of life
Adverse events
Data Extraction
We extracted relevant data on study characteristics (i.e., study design, sample size, follow-up duration, reported outcomes, and outcome definitions) and risk-of-bias items. We summarized these data in tables.
Statistical Analysis
We did not pool the results of the studies because of the small number of studies included and the heterogeneous outcomes reported. Instead, we summarized the results in tables and described them in the text.
Quality of Evidence
The level of quality of the body of evidence for each outcome was evaluated according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Handbook.14 We started with the assumption that randomized controlled trials are high quality, whereas observational studies are low quality. We then rated the studies based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, publication bias, magnitude of effect, dose-response gradient, and any residual confounding factors. The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology. The quality level determination reflects our certainty about the evidence.
We assessed risk of bias using the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS), which included six domains: confounding, selection bias, measurement bias, publication bias, model misspecification, and other bias.15
Expert Consultation
We consulted with an vitreoretinal surgeon on the use of the Argus II retinal prosthesis system between February and June 2017 for this update. The role of the expert advisor was to provide advice on research questions, review methods and results, and contextualize the evidence for the effectiveness and safety of the Argus II retinal prosthesis system. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted expert.
Results
Literature Search
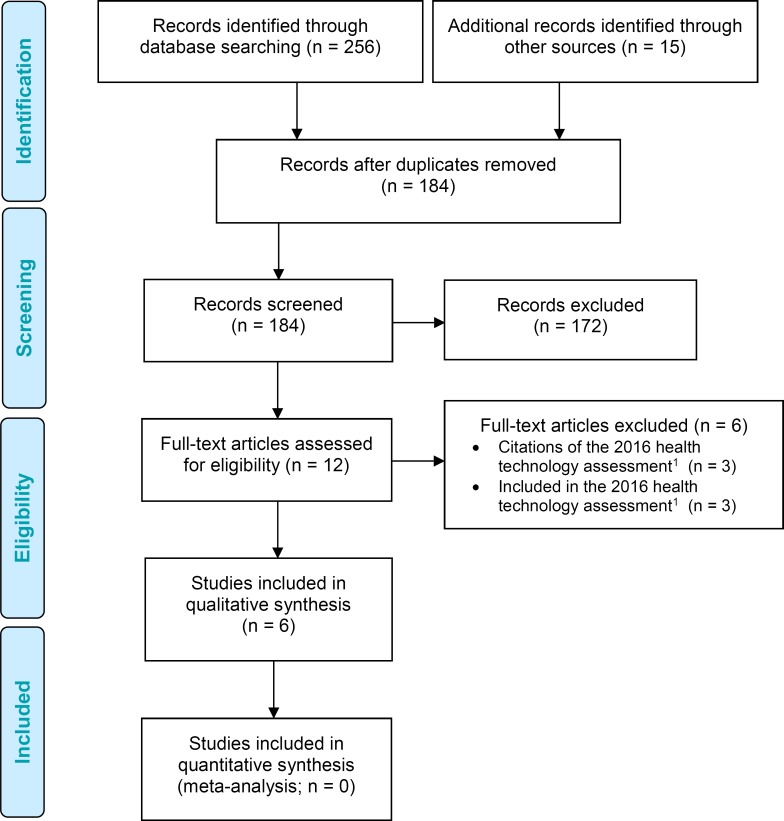
The literature search yielded 184 citations published between January 1, 2015, and February 9, 2017, after removing duplicates. We reviewed titles and abstracts to identify potentially relevant articles. We obtained the full texts of these articles for further assessment. Four studies (all observational) met the inclusion criteria. We hand-searched the reference lists of the included studies, along with health technology assessment websites and other sources, to identify additional relevant studies, but no citations were added.
Figure 1 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 1: PRISMA Flow Diagram for the Clinical Evidence Review.
Source: Adapted from Moher et al.16
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The literature search identified a guideline published by the United Kingdom National Institute for Health and Care Excellence (NICE) in 2015,17 a technology assessment published by the United States Agency for Healthcare Research and Quality (AHRQ) in 2016,18 and four primary studies.
The NICE guideline17 included two recommendations. First, it noted that current evidence on the safety and efficacy of the epiretinal prosthesis for retinitis pigmentosa is limited in quality and quantity, and that the implant procedure should be used only in the context of research. Second, NICE encouraged further research into this technology, with outcomes such as impact on quality of life and activities of daily living, and the durability of the implants. The guideline may be updated following publication of further evidence.
The AHRQ technology assessment18 evaluated the safety and efficacy of all retinal prosthesis systems for retinitis pigmentosa and age-related macular degeneration, including the Argus II system. The studies it reviewed on the Argus II system were evaluated in the 2016 health technology assessment1 or will be reviewed in this update.8,19 As a result, we will not discuss the AHRQ technology assessment in this report.
All four primary studies published findings from the Argus II International Study, a prospective, single-arm, non-randomized clinical study. The study design and inclusion criteria are described in the 2016 health technology assessment.1 One study presented the 5-year follow-up results for the predefined efficacy and safety outcomes.19 Two studies described the 3-year follow-up results for observer-rated functional vision8 and vision-related quality of life.20 One study described the performance of real-world functional vision tasks over a follow-up period of 6 to 36 months.21 Table 1 summarizes the characteristics of the four included primary studies.
Table 1:
Characteristics of Studies on the Argus II Retinal Prosthesis System
| Author, Year | Sample Size, n | Follow-up Period | Outcomes |
|---|---|---|---|
| da Cruz et al, 201619a | 27 21 |
60 months |
|
| Geruschat et al, 20168b | 26 | 18–44 months (mean 36 months) |
|
| Dagnelie et al, 201621 | 26 | 6–36 months |
|
| Duncan et al, 201620c | 9–20 | 12/18/24/36 months |
|
Results for Visual Function
Table 2 presents the findings for visual function. The results from the 1- and 3-year follow-up were reported in the 2016 health technology assessment1 and are presented here for comparison. Results are expressed as the percentage of patients who performed significantly better with the Argus II system on versus off.
Table 2:
Visual Function
| Author, Year | 1 Year | 3 Years | 5 Years |
|---|---|---|---|
| Object Localizationa | |||
| Ho et al, 201524 | 93.8% (P < .05) | 89.3% (P < .05) | — |
| da Cruz et al, 201619 | — | — | 80.9% (P < .05) |
| Direction of Motionb | |||
| Ho et al, 201524 | 62.5% (P < .05) | 55.6% (P < .05) | — |
| da Cruz et al, 201619 | — | — | 50.0% (P < .05) |
| Grating Visual Acuityc | |||
| Ho et al, 201524 | 48.2% (P < .05) | 33.3% (P < .05) | — |
| da Cruz et al, 201619 | — | — | 38.1% (P < .05) |
Patients to locate and touch a white square in random locations on a black monitor. Response error was measured by the distance (in cm) between the patient's touch and the square centre.
Patients to draw the path of a white line moving across a black monitor. Response error was measured by the difference (in degrees) between the response angle and the target bar angle.
Patients to differentiate the orientation of black and white bars with different widths. Results indicated the percentage of patients who scored between 2.9 and 1.6 logMAR with the system on.
At the 5-year follow-up, with the Argus II system on, most patients continued to perform significantly better on object localization, and half of patients performed significantly better in detecting direction of motion.19 Although there was some numeric decline in the percentage of patients who performed significantly better with the Argus II system on in both tasks over time, it was unclear whether that difference was statistically significant.19,24 At no time point did patients score 2.9 logMAR or better with the Argus II system off. The percentage of patients who scored 2.9 logMAR or better with the Argus II system on was sustained over time.19,24
The quality of the evidence for visual function was moderate (Table 3).
Table 3:
GRADE Evidence Profile for Visual Function, Comparison of the Argus II Retinal Prosthesis System On and Off
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Object Localization | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Direction of Motion | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Grating Visual Acuity | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Observational studies started with a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding, and loss to follow-up). We did not lower the GRADE level further unless there were more substantial study limitations.
The natural history of retinitis pigmentosa is a progressive deterioration of vision, eventually leading to blindness. The Argus II retinal prosthesis system is the only treatment option currently available to restore partial functional vision for these patients.
Results for Functional Outcomes
Table 4 presents the findings for functional outcomes. The results from the 1- and 3-year follow-up were reported in the 2016 health technology assessment1 and are presented here for comparison. Unless otherwise stated, the results are expressed as the mean percentage of success on each task with the Argus II system on versus off.
Table 4:
Functional Outcomes
| Author, Year | 1 Year | 3 Years | 5 Years |
|---|---|---|---|
| Orientation and Mobility (Find the Door)a | |||
| Ho et al, 201524 | 53% vs. 31% (P < .05) | 54% vs. 19% (P < .05) | — |
| da Cruz et al, 201619 | — | — | 52% vs. 23% (P < .05) |
| Orientation and Mobility (Follow the Line)b | |||
| Ho et al, 201524 | 73% vs. 17% (P < .05) | 68% vs. 14% (P < .05) | — |
| da Cruz et al, 201619 | — | — | 66% vs. 17% (P < .05) |
| Visual Orientationc | |||
| Geruschat et al, 20168 | — | −1.36 ± 0.19 (−38%; P < .001) | — |
| Visual Mobilityc | |||
| Geruschat et al, 20168 | — | −0.82 ± 0.20 (−22%; P = .003) | — |
| Daily Lifec | |||
| Geruschat et al, 20168 | — | −0.58 ± 0.12 (−19%; P = .001) | — |
| Interaction With Othersc | |||
| Geruschat et al, 20168 | — | −0.79 ± 0.15 (−20%; P < .001) | — |
| Sock Sortingd | |||
| Dagnelie et al, 201621 | — | Felt cover: 72% ± 19% vs. 33% ± 12% (P < .01) Bare table: 54% ± 23% vs. 35% ± 8% (P < .01) |
|
| Sidewalk Trackinge | |||
| Dagnelie et al, 201621 | — | 4.9 ± 2.6 vs. 6.9 ± 3.0 (P < .05) | — |
| Walking Direction Discriminationf | |||
| Dagnelie et al, 201621 | — | 67% vs. 7% | — |
Patients to walk across a room and find a simulated door. Success was defined as being able to touch the door.
Patients to follow a white line on the floor. Success was defined as being able to end on the line at its end point.
Patients to complete the Functional Low-Vision Observer Rated Assessment (FLORA) instrument22 with 35 tasks grouped into four domains: visual orientation (6 tasks), visual mobility (5 tasks), daily life (17 tasks), and interaction with others (7 tasks). An observer rated the performance of the task using a four-point scale, with scores ranging from 4 (impossible) to 1 (easy). Results expressed in score ± standard error of the mean with the Argus II system on minus off. The percentage of change is presented in parentheses. A negative value or percentage represents an improvement in function.
Patients to sort socks in colour with varying lighting and table surfaces, including felt cover and bare table. Results are the percentages (± standard deviation) of socks correctly identified by their colours comparing the Argus II system on versus off.
Patients to detect and track edges in an outdoor situation where lighting and contrast conditions are uncontrolled. Results are the number of out-of-bound counts in mean ± standard deviation with the Argus II system on versus off.
Patients to identify the walking direction of people passing in front of them while sitting in a stationary position. Results are the percentages of patients with the number of correct answers significantly above chance (P < .05) with the Argus II system on versus off.
At the 5-year follow-up, patients performed significantly better on the door task and the line task when the Argus II system was on, similar to the 1-year and 3-year follow-up. The actual percentage of patients who performed significantly better on both tasks appeared to decline over time,19 but individual participant data are needed to determine whether the difference was statistically significant.
For observer-rated functional outcomes, 24 out of 35 tasks (69%) from the Functional Low-Vision Observer Rated Assessment (FLORA)22 were statistically easier to achieve with the Argus II system on. The orientation domain showed the largest improvement, and the daily life domain showed the smallest improvement.8
Dagnelie et al21 presented the results of three real-world functional vision tasks, including sock sorting, sidewalk tracking, and walking direction discrimination, which mimicked activities that patients perform in their daily lives. Patients as a group performed significantly better on all three tasks when the Argus II system was on.
The quality of the evidence for functional outcomes was moderate (Table 5).
Table 5:
GRADE Evidence Profile for Functional Outcomes, Comparison of the Argus II Retinal Prosthesis System On and Off
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Orientation and Mobility (Find the Door) | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Orientation and Mobility (Follow the Line) | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Visual Orientation | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Visual Mobility | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Daily Life | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Interaction With Others | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Sock Sorting | |||||||
| 1 (observational)21 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Sidewalk Tracking | |||||||
| 1 (observational)21 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Walking Direction Discrimination | |||||||
| 1 (observational)21 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Observational studies started with a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding, and loss to follow-up). We did not lower the GRADE level further unless there were more substantial study limitations.
The natural history of retinitis pigmentosa is a progressive deterioration of vision, eventually leading to blindness. The Argus II retinal prosthesis system is the only treatment option currently available to restore partial functional vision for these patients.
Results for Quality of Life
Table 6 presents the findings for vision-related quality of life. The dimensions for this outcome were derived from the Vision and Quality of Life Index (VisQoL)23, and scores were registered on a 5- or 6-point scale. A lower score reflected little to no effect on quality of life because of loss of vision.
Table 6:
Vision-Related Quality of Lifea
| Author, Year | Baseline Mean Survey Score | Follow-up Mean Survey Score | P-value |
|---|---|---|---|
| Injuryb | |||
| Duncan et al, 201620 | 3.8 | 2.8 | 0.036 |
| Lifec | |||
| Duncan et al, 201620 | 4.4 | 3.7 | 0.0069 |
| Assistanced | |||
| Duncan et al, 201620 | 3.1 | 2.4 | 0.18 |
| Rolese | |||
| Duncan et al, 201620 | 4.6 | 3.8 | 0.0012 |
| Activitiesf | |||
| Duncan et al, 201620 | 4.1 | 3.8 | 0.10 |
Scores were registered on a 5- or 6-point scale. A lower score reflected little to no effect on quality of life caused by loss of vision.
Patient to respond to the question, “Does my vision make it likely I will injure myself (that is, when moving around the house, yard, neighbourhood, or workplace)?”
Patient to respond to the question, “Does my vision make it difficult to cope with the demands in my life?”
Patient to respond to the question, “Do I have difficulty organizing any assistance I may need?”
Patient to respond to the question, “Does my vision make it difficult to fulfil the role I would like to fulfil in my life (for example, family roles, work roles, community roles?”
Patient to respond to the question, “Does my vision affect my confidence to join in everyday activities?”
The mean baseline VisQoL utility score of 0.62 was not significantly different from the mean utility scores at follow-up, which ranged from 0.63 to 0.67. However, Argus II implantation led to significant improvements on three dimensions of the VisQoL: injury, life (coping with life's demands), and roles (fulfilling life roles).20 No patients reported difficulty in the friendship dimension at baseline or follow-up.
The quality of evidence for quality of life was moderate for all outcomes except assistance Table 7).
Table 7:
GRADE Evidence Profile for Vision-Related Quality of Life, Comparison of the Argus II Retinal Prosthesis System On and Off
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Injury | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Life | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Assistance | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Nonec | ⊕⊕ Low |
| Roles | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Activity | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Observational studies started with a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding, and loss to follow-up). We did not lower the GRADE level further unless there were more substantial study limitations.
The natural history of retinitis pigmentosa is a progressive deterioration of vision, eventually leading to blindness. The Argus II retinal prosthesis system is the only treatment option currently available to restore partial functional vision for these patients.
Not upgraded for the assistance domain because of large standard error and small sample size (i.e., imprecision).
Results for Adverse Events
The 2016 health technology assessment1 reported serious adverse events at 1 year and 3 years after implantation, but since the 3-year follow-up, only one additional severe adverse event has been reported. One patient experienced a retinal detachment in the implanted eye approximately 4.5 years after implantation.19
At 5 years after Argus II implantation, 60% of patients (18/30) had experienced no device- or surgery-related severe adverse events. A total of 24 severe adverse events were reported in the remaining 12 patients; all were treated with standard ophthalmic approaches. In the studies, no patients lost eyes or had damaged residual vision. Three Argus II systems were explanted, at 14 months, 3.5 years, and 4.3 years after implantation. Of the remaining 27 patients, 24 have functional implants.19
Discussion
The 5-year follow-up data from the Argus II International Study demonstrated the long-term efficacy and safety of the system as a means of restoring partial functional vision to patients with advanced retinitis pigmentosa. Changes in device design, revisions to surgical techniques, and upgrades to software were made to improve patient outcomes based on preliminary results and applied throughout the Argus II International Study.19,24
Given the rarity of retinitis pigmentosa, its clinical presentation, and the difficulties associated with conducting research in this population, there were inherent limitations in the evidence, such as low statistical power from a small sample size, inability to mask or randomize treatment, and the lack of objective measures to quantify functional gains.
Based on the most recent efficacy and safety data from the Argus II International Study, jurisdictions including the United States, the United Kingdom, and Germany have approved funding of this technology to restore some basic visual function to patients with advanced retinitis pigmentosa.
Conclusions
Based on evidence of moderate quality, the Argus II retinal prosthesis system significantly improved visual function, real-life functional outcomes, and quality of life in patients with profound vision loss from advanced retinitis pigmentosa. These improvements appeared to be sustained over time and had an acceptable safety profile.
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of the Argus II retinal prosthesis system compared with standard care in patients with retinitis pigmentosa?
Methods
Literature Search
We performed an updated economic literature search on February 9, 2017, for studies published from January 2015 to February 9, 2017. The previous economic literature search included studies prior to May 2015. To retrieve relevant studies, the search was developed using the clinical search strategy with an economic filter applied.
Database auto-alerts were created in MEDLINE and Embase and monitored for the duration of the health technology assessment review. We performed targeted grey literature searching of health technology assessment agency sites and clinical trial registries. Finally, we reviewed the reference lists of included economic literature for any additional relevant studies not identified through the systematic search. See Clinical Evidence, Literature Search, above, for methods used, and Appendix 1 for literature search strategies, including all search terms.
Literature Screening
A single reviewer reviewed titles and abstracts, and, for those studies meeting the eligibility criteria, we obtained full-text articles.
Types of Studies
We looked at full economic evaluations, such as cost-utility analyses, cost-effectiveness analyses, and cost-benefit analyses. We looked at economic evaluations reporting incremental cost-effectiveness ratios (ICERs; e.g., cost per quality-adjusted life-year [QALY]/life-years gained or cost per event avoided).
Types of Participants
The population of interest was patients with retinitis pigmentosa.
Types of Interventions
The intervention of interest was the Argus II retinal prosthesis system.
Types of Outcomes Measures
Outcomes of interest were costs, QALYs, incremental cost, incremental effectiveness, and cost per QALY gained.
Data Extraction
We extracted relevant data on the following:
Source (i.e., name, location, year)
Population and comparator
Interventions
Outcomes (i.e., health outcomes, costs, and ICERs)
We contacted authors of the studies to provide clarification as needed.
Study Applicability
We determined the usefulness of each identified study for decision-making by applying a modified applicability checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. The original checklist is used to inform development of clinical guidelines by NICE. We retained questions from the NICE checklist related to study applicability and modified the wording of the questions to remove references to guidelines and to make it Ontario-specific. A summary of the number of studies judged to be directly applicable, partially applicable, or not applicable to the research question is presented.
Results
Literature Search
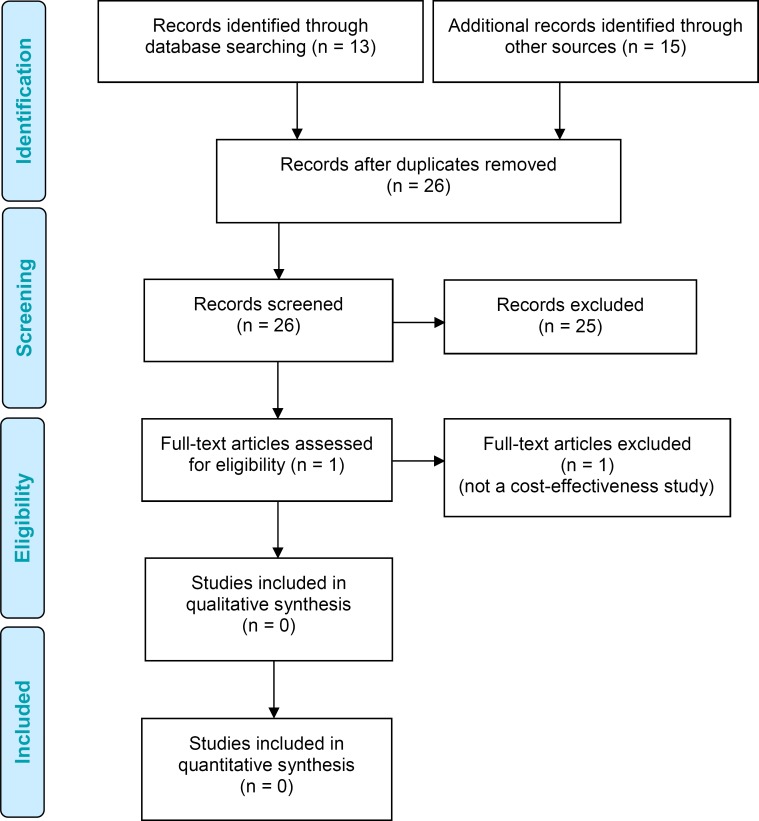
The literature search yielded 26 citations published between January 2015 and February 9, 2017, (with duplicates removed). We excluded a total of 25 articles based on information in the title and abstract. We then obtained the full text of one potentially relevant article for further assessment. Figure 2 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 2: PRISMA Flow Diagram for the Economic Evidence Review.
Source: Adapted from Moher et al.16
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Review of Included Economic Studies
Since the publication of 2016 health technology assessment on the Argus II system,1 we identified no new economic studies on the use of the Argus II system to treat retinitis pigmentosa.
Conclusions
We identified no new cost-effectiveness or cost-utility studies on the Argus II system to treat retinitis pigmentosa.
PRIMARY ECONOMIC EVALUATION
Based on the findings of the 2016 health technology assessment,1 the Ontario Health Technology Advisory Committee recommended against public funding of the Argus II and suggested that Health Quality Ontario re-evaluate the effectiveness of the system in 1 year.2 The objective of this analysis was to evaluate the new evidence on the cost-effectiveness of the Argus II system in treating patients with bare to no light perception vision from advanced retinitis pigmentosa to determine whether it should be publicly funded.
Research Question
What is the cost-effectiveness of the Argus II retinal prosthesis system compared with standard care in patients with retinitis pigmentosa within the context of the Ontario Ministry of Health and Long-Term Care?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards Statement.25
Type of Analysis
We conducted a cost-utility analysis to estimate the annual costs and health outcomes (i.e., QALYs) of the Argus II system.
Target Population
The study population was men and/or women aged 45 years and older presenting with retinitis pigmentosa, a hereditary genetic disease that causes bilateral retinal degeneration.
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care.
Interventions
We conducted evaluations of the Argus II retinal prosthesis system compared with standard care (i.e., rehabilitation or nursing).
Discounting and Time Horizon
In accordance with revised guidelines from the Canadian Agency for Drugs and Technologies in Health, we applied annual discount rates of 1.5% in the base case analysis for both costs and QALYs. We also conducted a scenario analysis with a discount rate of 5%.26,27 We used a 20-year time horizon in the base case analysis and a 10-year time horizon in the scenario analysis.
Model Structure/Structure of Analysis
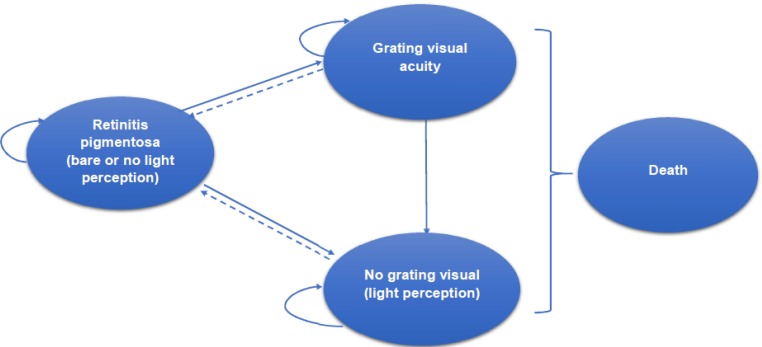
We applied a Markov cohort model, developed for the 2016 health technology assessment,1 to capture visual function—namely grating visual acuity (GVA) or no grating visual acuity (NGVA)—in retinitis pigmentosa patients fitted with the Argus II implant.
Grating visual acuity was defined as reliably achieving scores of 2.9 and 1.6 logMAR on the scale of visual acuity with the Argus II system on.
Details of the original model are described in the 2016 health technology assessment.1 However, based on clinical evidence from a 5-year follow-up by da Cruz et al,19 we made several major changes in the current assessment:
Assuming that vision is sustained after the third year of Argus II implantation, patients with retinitis pigmentosa who achieved GVA remained in that state. In the previous model, patients who achieved GVA after Argus II implantation could move to NGVA
In the base case analysis, the current model followed a cohort for 20 years. The previous model used a time horizon of 10 years
The current model captured mortality in patients with retinitis pigmentosa; the previous model did not
The age of patients in the current model is 45 years and older due to changes in patient selection criteria; in the previous model, patients were 50 years and older.
Figure 3 shows a schematic diagram of the Markov model for Argus II implantation in patients with retinitis pigmentosa.
Figure 3: Markov Model for Argus II Implantation in Patients With Retinitis Pigmentosa.
Note: The dashed lines show Argus II explantation.
Clinical Outcome and Utility Parameters
Transition Probabilities
To determine transition probabilities (Table 8), we applied the 3- and 5-year published clinical results from a controlled, non-randomized, prospective, multicentre study conducted in 10 sites in Europe and in the United States.19,24,28
Table 8:
Model Variable Inputs Used in the Base Case Analysis
| Model Parameters | Base Case Value | Range | Reference |
|---|---|---|---|
| Probability of severe adverse events resulting from Argus II implantation in the first year | 0.3333 | 0.2499–0.4166 | Humayun et al, 201228 |
| Annual probability of severe adverse events between years 1 and 3 | 0.0465 | 0.0349–0.0581 | Ho et al, 2015,24,28 and Humayun et al, 2012,28 plus calculation |
| Annual probability of severe adverse events between years 3 and 5 | 0.04257 | 0.0319–0.0532 | da Cruz et al, 201619 |
| Probability of patients achieving GVA in the first year after Argus II implantation | 0.4820 | 0.3615–0.6025 | Humayun et al, 201228 |
| Annual probability of patients moving from GVA to NGVA between years 1 and 3 | 0.1688 | 0.1266–0.2110 | Ho et al, 2015,24,28 and Humayun et al, 2012,28 plus calculation |
| Probability of Argus II explantation in year 1 | 0.0333 | 0.0249–0.0416 | Humayun et al, 201228 |
| Probability of Argus II explantation in year 2 | 0.0347 | — | da Cruz et al, 201619 |
| Probability of Argus II explantation in year 3 | 0.0351 | — | da Cruz et al, 201619 |
| Standardized mortality ratio, patients with retinitis pigmentosa vs. general population | 1.56 | 1.28–2.61 | Na et al, 201730 |
| Mortality from the Canadian life-table | Life-table | — | Statistics Canada31 |
| Discount rate | 1.5% | 0%–5% | CADTH26,27 |
Abbreviations: CADTH, Canadian Agency for Drugs and Technologies in Health; GVA, grating visual acuity; NGVA, no grating visual acuity.
These studies reported primary outcomes for visual function using three different visual acuity tests: square localization, direction of motion, and GVA.19,24,28 Similar to the 2016 health technology assessment,1 we selected GVA as the visual outcome for the Markov model, because we could assign utility weights for patients who did or did not achieve GVA based on expert consultation and the literature. We used clinical data from years 1 and 3 to calculate the yearly vision transition probability for the first three years after Argus II implantation, using a formula reported elsewhere.29 Clinical data from years 3 to 5 showed that vision improvement was sustained in patients who received an Argus II implant. Therefore, we assumed that after year 3, there would be no probability of patients moving from GVA to NGVA. Using the available clinical data, we assumed that patients implanted with the Argus II system could not move from NGVA to GVA. We assumed the yearly visual transition probability to be constant for the rest of the time horizon.
Argus II Explantation Probability
Data from the study described above showed that three patients had the device removed due to severe adverse events, at 1.2 years, 3.5 years, and 4.3 years.19,24,28 Because there are no clinical data beyond 5 years after Argus II implantation, we assumed that no explantations would take place after 5 years. Once the device was explanted, the patient would return to the retinitis pigmentosa state in the model.
Utilities
The utility values for health states of retinitis pigmentosa, GVA, and NGVA are presented in Table 9. Detailed information describing the calculations are reported in the 2016 health technology assessment.1
Table 9:
Utilities Used in the Economic Model
| Health State | Base Case Value | Range | Reference |
|---|---|---|---|
| Retinitis pigmentosa, no light perception | 0.26 | 0.19–0.33 | Brown et al, 200132 |
| NGVA, light perception | 0.35 | 0.33–0.60 | Brown et al, 199933 |
| GVA | 0.52 | 0.36–0.68 | Brown et al, 199933 |
Abbreviations: GVA, grating visual acuity; NGVA, no grating visual acuity.
Cost Parameters
Costs included those for the device, the procedure, and maintenance. Table 10 summarizes the main cost parameters for the cost-effectiveness model. The University Health Network provided the information. Appendix 3 provides a detailed breakdown of the unit costs related to the Argus II surgery.
Table 10:
Costs Used in the Economic Model
| Variable | Base Case Value | Range | Reference |
|---|---|---|---|
| Cost of Argus II device | $179,850 | $134,888–$224,813 | UHNa |
| Cost of Argus II device and implantation | $195,906 | $146,930–$244,883 | UHNa |
| Cost of Argus II annual device maintenance | $8,270 | $6,203–$10,338 | UHNa |
| Annual cost of treatment for patients who received standard care | $17,727 | $13,295–$22,158 | Frick et al, 201234 |
| Annual cost of treatment for patients who did not achieve GVA after the Argus II implant | $14,756 | $11,067–$18,445 | Frick et al, 2012,34 plus assumptions |
| Annual cost of treatment for patients who achieved GVA after the Argus II implant | $11,786 | $8,839–$14,732 | Frick et al, 2012,34 plus assumptions |
| Annual cost of treatment for severe adverse events | $333 | $250–$416 | UHNa |
| Cost of Argus II device explantation | $5,042 | $3,737–$6,347 | UHNa |
Abbreviations: GVA, grating visual acuity; UHN, University Health Network.
Cost data were provided in the submission for the review of Argus II (expert opinion).
Since the 2016 health technology assessment,1 the cost of the Argus II system, according to the submission we received, has decreased by $19,862, from $199,712 per system in 2015 to $179,850 in 2017.
Similar to the 2016 health technology assessment,1 we took the annual treatment cost for patients who did not receive Argus II implants from a study by Frick et al,34 the only published study on treatment costs for patients with retinitis pigmentosa thus far. Costs from Frick et al34 were reported in 2012 US dollars; we converted them to 2012 Canadian dollars using an exchange rate of 1 US dollar = 1.01 Canadian dollars reported by the Bank of Canada,35 and then inflated to 2017 values using the consumer price index.36 Details of cost calculations and assumptions were identical to the 2016 health technology assessment.1 We also extracted costs for treatment of severe adverse events from the 2016 health technology assessment.
Analysis
The primary outcome of the base case analysis was ICERs or costs and QALYs comparing the Argus II system with standard care. We calculated ICERs by taking the difference in expected costs between the Argus II system and standard care, divided by the difference in expected QALYs produced by these two interventions.
We assessed the variability and uncertainty of model parameters by conducting one-way, scenario, and probabilistic sensitivity analyses.
For the one-way sensitivity analyses, we varied specific model variables over plausible ranges and examined the impact on ICERs. The results of the one-way sensitivity analyses are presented in a tornado diagram.
We conducted scenario analyses to explore differences in ICERs by changing the time horizon in the base case from 20 years to 10 years and by changing the discount rate in the base case from 1.5% to 5%.
To determine the effect of simultaneously varying numerous variables within the assigned distributions, we conducted a probabilistic sensitivity analysis by running 1,000 simulations of the model parameters. We applied beta distributions to probabilities and utility parameters. We applied gamma distributions to cost parameters. We applied lognormal distribution to the standardized mortality ratio, comparing patients with retinitis pigmentosa to the general population. The results of the probabilistic sensitivity analysis are shown using a cost-effectiveness acceptability curve.
Main Assumptions
The major assumptions we made for this model were:
Patients would achieve the best vision improvement one year after an Argus II implant
Between years 1 and 3 after Argus II implantation, patients who achieved GVA could move to NGVA
After three years with an Argus II implant, patients who achieved GVA would remain in GVA
After an Argus II implant, patients in NGVA would stay in NGVA
If an Argus II device was extracted, the patient would return to the retinitis pigmentosa state
Generalizability
The findings of this economic analysis cannot be generalized to all patients with retinitis pigmentosa. They may, however, be used to guide decision-making about the specific patient populations addressed in the studies included in this health technology assessment.
Expert Consultation
We consulted an ophthalmologist on the use of the Argus II retinal prosthesis system between February and May 2017. The role of the expert advisor was to provide advice on research questions, review methods and results, and contextualize the evidence on the effectiveness and safety of the Argus II retinal prosthesis system. However, the statements, conclusions, and views expressed in this report do not necessarily represent the view of the consulted expert.
Results
Base Case Analysis
The results of the base case analysis are shown in Table 11.
Table 11:
Base Case Analysis Results
| Strategy | Average Total Cost | Incremental Costa | Average Total Effect | Incremental Effectb | ICERc |
|---|---|---|---|---|---|
| Standard care | $287,458 | — | 4.2161 | — | — |
| Argus II | $537,734 | $250,276 | 6.7849 | 2.5688 | $97,429 |
Abbreviations: ICER, incremental cost-effectiveness ratio.
Incremental cost = average total cost (Argus II) – average total cost (standard care).
Incremental effect = average total effect (Argus II) – average total effect (standard care).
ICER = incremental cost/incremental effect.
Sensitivity Analysis
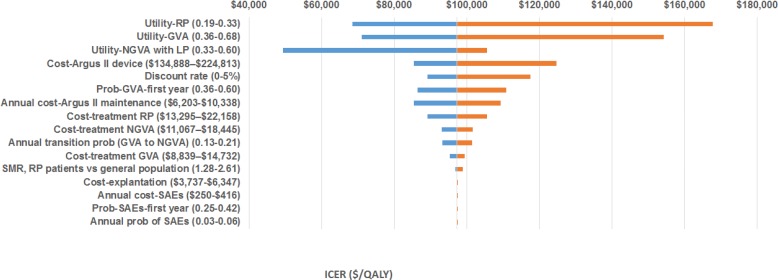
One-Way Sensitivity Analysis
The results of the one-way sensitivity analysis are presented in Figure 4. The model was most sensitive to the health-related utility of patients with retinitis pigmentosa; the health-related utility of patients who achieved GVA; the health-related utility of patients who did not achieve GVA but did achieve light perception; and the cost of the Argus II device.
Figure 4: One-Way Sensitivity Analysis: Argus II Versus Standard Carea.
Abbreviation: GVA, grating visual acuity; ICER, incremental cost-effectiveness ratio; LP, light perception; NGVA, no grating visual acuity; QALY, quality-adjusted life-year; RP, retinitis pigmentosa; SAE, severe adverse event; SMR, standardized mortality ratio.
aX-axis represents range of ICERs when base case values are varied (ranges shown in parentheses). Vertical line represents the ICER for the Argus II system ($97,429 per QALY gained).
Scenario Analysis
The findings of the scenario analyses are presented in Tables 12 and 13.
Table 12:
Scenario Analysis Results for a 10-Year Time Horizon (1.5% Discount Rate)
| Strategy | Average Total Cost | Incremental Costa | Average Total Effect | Incremental Effectb | ICERc |
|---|---|---|---|---|---|
| Standard care | $160,889 | — | 2.3597 | — | — |
| Argus II | $382,754 | $221,865 | 3.9209 | 1.5612 | $142,112 |
Abbreviations: ICER, incremental cost-effectiveness ratio.
Incremental cost = average total cost (Argus II) – average total cost (standard care).
Incremental effect = average total effect (Argus II) – average total effect (standard care).
ICER = incremental cost/incremental effect.
Table 13:
Scenario Analysis Results for a 5% Discount Rate (20-Year Time Horizon)
| Strategy | Average Total Cost | Incremental Costa | Average Total Effect | Incremental Effectb | ICERc |
|---|---|---|---|---|---|
| Standard care | $214,391 | — | 3.1444 | — | — |
| Argus II | $448,202 | $233,811 | 5.1289 | 1.9845 | $117,819 |
Abbreviations: ICER, incremental cost-effectiveness ratio.
Incremental cost = average total cost (Argus II) – average total cost (standard care).
Incremental effect = average total effect (Argus II) – average total effect (standard care).
ICER = incremental cost/incremental effect.
Probabilistic Sensitivity Analysis
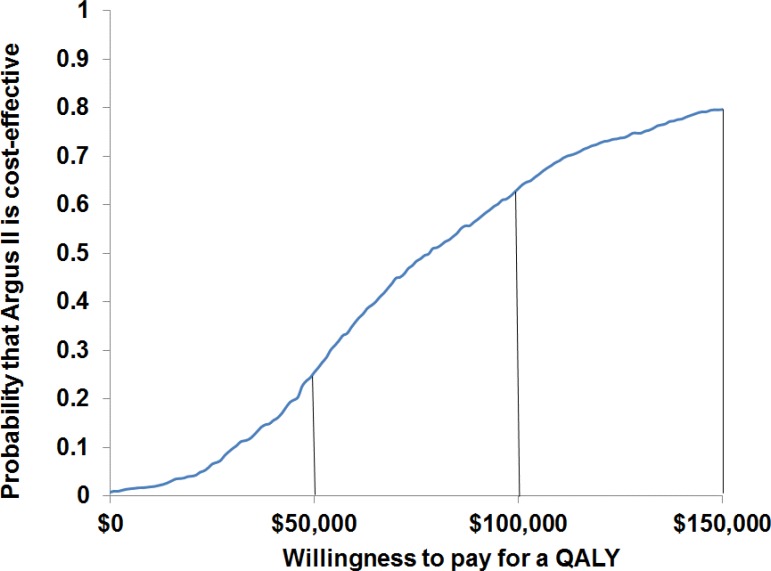
We ran a total of 1,000 simulations of the decision-analytic model comparing the Argus II system with standard care, using random draws of all model parameters within the assigned distributions. Results are presented in Figure 5. Assuming willingness-to-pay thresholds of $50,000, $100,000, and $150,000 per QALY, there was a 24%, 63%, and 79% chance, respectively, that the Argus II system would be cost-effective.
Figure 5: Cost-Effectiveness Acceptability Curve, Argus II Versus Standard Care.
Abbreviation: QALY, quality-adjusted life-year
Limitations
In the absence of data on the Argus II system after 5 years, we assumed that there would be no more explantations.
Discussion
In this update, the base case ICER for the Argus II system compared with standard care, at $97,432 per QALY gained, was substantially less than the $207,616 per QALY gained we found in the previous report.
The difference we found in this update has a number of explanations. First, the improved vision of patients with retinitis pigmentosa who received an Argus II implant was largely sustained, whereas earlier findings reported in the 2016 health technology assessment1 assumed greater worsening over time. Because of this, in the previous report we assumed that a proportion of patients who achieved GVA would move to NGVA; we changed this assumption in the model for the current analysis. Second, the price of the Argus II system is now $19,862 lower than it was when we conducted the 2016 health technology assessment.1 Third, we applied a discount rate of 1.5% instead of 5% in the base case analysis. Finally, the model in this update used a 20-year time horizon rather than a 10-year time horizon. We felt this was a reasonable time horizon given the longer-term follow up data now available.
Using the longer follow-up clinical data, the scenario analyses showed that even when we applied a 5% discount rate or a 10-year time horizon, the ICER values comparing the Argus II system with standard care were still lower ($142,112 per QALY gained with a 10-year time horizon and a 1.5% discount rate; $117,819 per QALY gained with a 5% discount rate and a 20-year time horizon) than reported in the 2016 health technology assessment ($207,616 per QALY gained).1
In this update, the probabilistic sensitivity analyses showed that at a willingness-to-pay threshold of $100,000 per QALY, there was a 63% chance that the Argus II system would be cost-effective, compared to a 21% chance in the 2016 health technology assessment.1
Conclusions
The base case analysis showed that compared with standard care, gaining a QALY with the Argus II system would require an additional $97,429 over a 20-year time horizon. Sensitivity analyses showed that the model parameters were robust.
BUDGET IMPACT ANALYSIS
We conducted a budget impact analysis from the perspective of the Ontario Ministry of Health and Long-Term Care to determine the estimated cost burden of the Argus II system over the next 5 years. All costs are reported in 2017 Canadian dollars.
Research Question
What would it cost the Ontario Ministry of Health and Long-Term Care to fund the Argus II system over the next 5 years?
Methods
Target Population
The target population was patients with retinitis pigmentosa who were eligible for Argus II implantation.
Resource
The Argus II retinal prosthesis is a novel technology that requires surgery. Only one centre in Ontario (University Health Network) performs the procedure. According to the data provided by the University Health Network for the Argus II system, four Argus II implants would be performed each year in Ontario (expert opinion). Table 14 provides the number of patients who would receive Argus II implants between 2017 and 2021.
Table 14:
Number of Patients Expected to Receive Argus II Implants in Ontario, 2017 to 2021
| Year | Patients per Year Post-implant | Total Patients, n | ||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||
| 2017 | 4 | 4 | ||||
| 2018 | 4 | 4 | 8 | |||
| 2019 | 4 | 4 | 4 | 12 | ||
| 2020 | 4 | 4 | 4 | 4 | 16 | |
| 2021 | 4 | 4 | 4 | 4 | 4 | 20 |
Canadian Costs
Except for the treatment costs for standard care (which were assumed to be the same as those for patients who did not receive the Argus II implant in a published study34), all costs used in the budget impact analysis were Ontario-specific and provided by the University Health Network (expert opinion). All costs were expressed in 2017 Canadian dollars.
We calculated budget impact based on the estimated number of Argus II implants to be done at the University Health Network over the next 5 years (2017 to 2021), using the cost of the Argus II device and surgery for each new implant, plus the annual maintenance cost. Cost details are provided in Table 15. A detailed breakdown of costs is provided in Appendix 3.
Table 15:
Costs for Argus II Implantation
| Resource Items | Cost | Source |
|---|---|---|
| Argus II device cost | $181,985a | UHNb |
| Argus II surgery cost | $3,808c | UHNb |
| Argus II labour cost | $10,113d | UHNb |
| Argus II device annual maintenance cost | $8,271e | UHNb |
Abbreviation: UHN, University Health Network.
Includes cost of Argus II device and Argus II training kits.
Cost data were provided in the resubmission for the review of Argus II by the University Health Network (expert opinion; Appendix 3).
Includes all surgery-related costs.
Includes all non-surgical costs.
Includes annual maintenance costs and cost to replace Argus II implant parts each year.
We assumed that in the year the system was implanted, only the device and surgery costs would be incurred, and that maintenance and treatment costs would be incurred in subsequent years. Costs were taken from the cost-effectiveness model (Table 10). Average costs per patient per year (Argus II implant and standard care) are presented in Table 16.
Table 16:
Average Cost Per Retinitis Pigmentosa Patient Per Year
| Therapy | Year Post-implant | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Argus IIa | $196,016c | $21,605d | $21,584 | $21,800 | $21,578 |
| Standard careb | $17,727 | $17,661 | $17,590 | $17,512 | $17,429 |
Abbreviation: GVA, grating visual acuity; NGVA, no grating visual acuity.
Cost data were provided in the resubmission for the review of Argus II by the University Health Network. Costs decreased over time for both Argus II and standard care as a result of mortality from conditions related and unrelated to retinitis pigmentosa.
Source: Frick et al, 2012.34 Costs in the first year and subsequent years included treatment costs for patients with retinitis pigmentosa.
Costs in the first year of Argus II implantation included the following: the Argus II device; health care labour, including rehabilitation, pre- and postoperative eye exams, Argus II system activation and fitting; surgical procedures, including instruments and supplies; and severe adverse events (if incurred). The maintenance cost was not included in the first year cost. A detailed breakdown of the costs is shown in Appendix 3.
Costs in the years following Argus II implantation included the following: Argus II maintenance; treatment for patients achieving NGVA; treatment for patients achieving GVA; severe adverse events (if incurred); and Argus II explanation (if incurred). Costs were calculated based on the proportion of patients who achieved NGVA and GVA after Argus II implant.
Analysis
We calculated the required budget to fund four Argus II implants per year. We also calculated the net budget impact (net cost) as the difference between the cost of the Argus II system if it were funded and the costs for standard care if patients did not receive the Argus II implant. We also conducted a one-way sensitivity analysis by reducing the price of the Argus II system by 25% per device.
Expert Consultation
We consulted an ophthalmologist on the use of the Argus II retinal prosthesis system between February and May 2017. The role of the expert advisor was to provide advice on research questions, review methods, and review results for the budget impact analysis. However, the statements, conclusions, and views expressed in this report do not necessarily represent the view of the consulted expert.
Results
Base Case Analysis
The expected budget impact of Argus II implantation for the next five years is presented in Table 17.
Table 17:
Budget Impact of Adopting the Argus II System in Ontario, 2017 to 2021
| Year | Strategy | Cost per Year Post-implant, $ | Total, $ | ||||
|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |||
| 2017 | Argus II | $784,064 | — | — | — | — | $784,064 |
| Standard care | $70,908 | — | — | — | — | $70,908 | |
| Net budget impact | $713,156 | — | — | — | — | $713,156 | |
| 2018 | Argus II | $784,064 | $86,422 | — | — | — | $870,485 |
| Standard care | $70,908 | $70,645 | — | — | — | $141,553 | |
| Net budget impact | $713,156 | $15,777 | — | — | — | $728,933 | |
| 2019 | Argus II | $784,064 | $86,422 | $86,336 | — | — | $956,821 |
| Standard care | $70,908 | $70,645 | $70,359 | — | — | $211,912 | |
| Net budget impact | $713,156 | $15,777 | $15,976 | — | — | $744,909 | |
| 2020 | Argus II | $784,064 | $86,422 | $86,336 | $87,198 | — | $1,044,019 |
| Standard care | $70,908 | $70,645 | $70,359 | $70,050 | — | $281,962 | |
| Net budget impact | $713,156 | $15,777 | $15,976 | $17,148 | — | $762,057 | |
| 2021 | Argus II | $784,064 | $86,422 | $86,336 | $87,198 | $86,310 | $1,130,330 |
| Standard care | $70,908 | $70,645 | $70,359 | $70,050 | $69,714 | $351,676 | |
| Net budget impact | $713,156 | $15,777 | $15,976 | $17,148 | $16,596 | $778,653 | |
Note: Numbers may appear inexact due to rounding.
Sensitivity Analysis
Table 18 presents the results of the sensitivity analysis, reflecting a 25% decrease in the price of the Argus II system.
Table 18:
Budget Impact of Adopting the Argus II System in Ontario, 2017 to 2021, When the Price Is Reduced by 25%
| Year | Strategy | Cost per Year Post-implant, $ | Total, $ | ||||
|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |||
| 2017 | Argus II | $604,216 | — | — | — | — | $604,216 |
| Standard care | $70,908 | — | — | — | — | $70,908 | |
| Net budget impact | $533,308 | — | — | — | — | $533,308 | |
| 2018 | Argus II | $604,216 | $86,422 | — | — | — | $690,637 |
| Standard care | $70,908 | $70,645 | — | — | — | $141,553 | |
| Net budget impact | $533,308 | $15,777 | — | — | — | $549,085 | |
| 2019 | Argus II | $604,216 | $86,422 | $86,336 | — | — | $776,973 |
| Standard care | $70,908 | $70,645 | $70,359 | — | — | $211,912 | |
| Net budget impact | $533,308 | $15,777 | $15,976 | — | — | $565,061 | |
| 2020 | Argus II | $604,216 | $86,422 | $86,336 | $87,198 | — | $864,171 |
| Standard care | $70,908 | $70,645 | $70,359 | $70,050 | — | $281,962 | |
| Net budget impact | $533,308 | $15,777 | $15,976 | $17,148 | — | $582,209 | |
| 2021 | Argus II | $604,216 | $86,422 | $86,336 | $87,198 | $86,310 | $950,482 |
| Standard care | $70,908 | $70,645 | $70,359 | $70,050 | $69,714 | $351,676 | |
| Net budget impact | $533,308 | $15,777 | $15,976 | $17,148 | $16,596 | $598,805 | |
Note: Numbers may appear inexact due to rounding.
Limitations
A limitation of this analysis was the absence of information about treatment costs for retinitis pigmentosa (other than the Argus II system) from an Ontario context. The treatment costs for retinitis pigmentosa used in this analysis may be higher than in reality; as a result, the findings of this analysis may be an overestimate.
Discussion
Since the 2016 health technology assessment1 was completed, the price of the Argus II system has decreased by approximately $20,000 per device (a 10% reduction). This price reduction might affect the budget required to fund the Argus II system, depending on the number of Argus II implants performed each year. Indeed, in the 2016 health technology assessment,1 the required budget ranged from $800,404 and $837,596 per year from 2015 to 2019; using the new lower price, the required budget in this update is slightly lower. The price of the Argus II system is an important factor in determining the budget for funding this novel technology.
Conclusions
If the Argus II system were publicly funded in Ontario in patients with retinitis pigmentosa, and if four implants were performed per year at one centre, we estimate that the net budget impact would be $0.71 million to $0.78 million per year over the next 5 years (2017 to 2021).
PUBLIC AND PATIENT ENGAGEMENT
Objective
The objective of this analysis was to explore the underlying values, needs, impacts, and preferences of those who have lived experience with retinitis pigmentosa. The treatment focus was the Argus II retinal prosthesis system.
Background
Public and patient engagement explores the lived experience of a person with a health condition, including the impact that the condition and its treatment has on the patient, as well as the patient's family or other caregivers, and the patient's personal environment. Public and patient engagement increases awareness and builds appreciation for the needs, priorities, and preferences of the individual at the centre of a treatment program.
Lived experience is a unique source of evidence about the personal impact of a health condition and how that condition is managed, including what it is like to navigate the health care system with that condition, and how technologies may or may not make a difference in people's lives. Information shared from lived experience can also identify gaps or limitations in published research (for example, outcome measures that do not reflect what is important to those with lived experience).37–39 Additionally, lived experience can provide information or perspectives on the ethical and social-values implications of technologies and treatments. Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored in the published literature, Health Quality Ontario sometimes reaches out to and directly speaks with people who live with the health condition, including those who may have experience with the intervention in question.
Methods
Engagement Plan
Engagement as a concept captures a range of efforts used to involve the public and patients in various domains and stages of health technology assessment decision-making.40 Rowe and Frewer outline three types of engagement: communication, consultation, and participation.41 Communication constitutes a one-way transfer of information from the sponsor to the individual, while participation involves the sponsor and individual collaborating through real-time dialogue. Consultation, on the other hand, refers to the sponsor seeking out and soliciting information (for example, experiential input) from the public, patients, and caregivers affected by the intervention in question.42
The engagement plan for this health technology assessment was consultation. Within this typology, the engagement design focused on an interview methodology to examine the lived experience of patients, including those who have the Argus II retinal prosthesis.
The qualitative interview was selected as an appropriate methodology, because it allowed Health Quality Ontario staff to deeply explore the meaning of central themes in the lived experience of the participants. The main task in interviewing is to understand the meaning of what participants say.43 Interviews are particularly useful for getting the story behind a participant's experiences, which was the objective of this part of the study. The sensitive nature of exploring quality-of-life issues is another reason supporting the use of interviews for this project.
Recruitment of Participants
The recruitment strategy for this project pursued an approach called purposive sampling44–47 to actively recruit individuals with direct lived experience. At the outset of this health technology assessment, we knew that very few Canadians had used the Argus II system. Attempts to recruit these individuals went through a hospital in Ontario that had established relationships with them.
To reach people who lived with retinitis pigmentosa, we contacted the Canadian National Institute for the Blind and found people willing to be interviewed who were at different stages of progression.
Inclusion Criteria
We sought patients with retinitis pigmentosa who may or may not have received the Argus II retinal prosthesis.
Exclusion Criteria
We set no exclusion criteria.
Participants
We spoke with patients who had a history of retinitis pigmentosa. Some patients had received the Argus II retinal prosthesis; others had not.
Approach
At the outset of the interview, we explained the purpose of the health technology assessment process (including the role of Health Quality Ontario and the Ontario Health Technology Advisory Committee), risks to participation, and protection of personal health information. These attributes were explained to individuals verbally and through a letter of information. Written consent was then obtained from participants prior to commencing the interview. The letter of information and consent form are attached as Appendix 4. Interviews were recorded and transcribed.
Questions focused on the impact of retinitis pigmentosa on quality of life, and on the person's experience of any other health interventions related to managing retinitis pigmentosa. During the interviews with the patients who received the Argus II system, we also asked questions about experiences with the procedure itself, any post-surgery rehabilitation, and perceived benefits or limitations of the technology. The interview guide is attached as Appendix 5.
The interview used a semi-structured approach, consisting of a series of open-ended questions. Interviews lasted approximately 45 to 60 minutes. Questions for the interview were based on a list developed by the Health Technology Assessment international (HTAi) Interest Group on Patient and Citizen Involvement in HTA (PCIG) to elicit lived experience specific to the impact of a health technology or intervention on lived experience and quality of life.48
Data Extraction and Analysis
We selected a modified version of a grounded theory methodology to analyze the transcripts of participant interviews, because it captures and allows for elements of the lived experience to be themed and compared across participants. The inductive nature of grounded theory follows an iterative process of eliciting, documenting, and analyzing responses while simultaneously collecting and analyzing data using a constant comparative approach.49,50 Through this approach, staff coded transcripts and compared themes using NVivo (QSR International, Doncaster, Victoria, Australia). NVivo enables the identification and interpretation of patterns in the interview data about the meaning and implications of the lived condition from the perspective of what was important in their daily lived experience with retinitis pigmentosa, before and after the intervention in question.
Results
Gradual but Persistent Progression of Disease
Retinitis pigmentosa progresses gradually, typically starting in childhood and proceeding into adulthood at a varying rate. Participants described the progressive nature of retinitis pigmentosa as both positive and negative: positive because they could adapt over time, but negative because there was no way to slow the progression. Patients reported occasionally feeling stigmatized, especially when they were younger, because their normal appearance caused some people to accuse them of pretending to be visually impaired.
It has been a very gradual deterioration. I can still see if the lights are on. I can tell if it's daylight or dark.
Impact of Retinitis Pigmentosa on Quality of Life
All participants said that retinitis pigmentosa had a substantial impact on their quality of life. The impact increased as the disease progressed and eyesight deteriorated. Participants were generally high-functioning and able to accomplish a variety of day-to-day tasks. All participants indicated that they relied on support to accomplish ordinary tasks. Often, patients reported having to rely on family members as caregivers. For people without family, this support took the form of technological devices or help from friends.
I have used a lot of aids. I have lots of adaptive technology. I [even] have a talking thermostat … almost everything I have talks.
Participants focused on the importance of planning, organizing, and adapting to their environment to meet their accessibility needs. They also spoke about how vision loss limited their mobility, restricted their access to information (print or online), and reduced their opportunities to forge a career path. They spoke about how retinitis pigmentosa made it more difficult to make life choices; for example, the need to live near accessible transit routes limited their choice of accommodation type and the communities where they could live.
Participants talked about how their attitude was a key determinant in overcoming challenges, being adept planners, and facing barriers. They described a strong will and determination as essential attributes:
Any barrier or any disability is very much a matter of attitude. I don't allow my vision loss to become something that defines restrictions for me in my life.
If you're a person who is not outgoing, resourceful, resilient, and the type of personality that lets barriers get in your way—if you're not obstinate, stubborn, and strong-willed— then vision loss is going to affect you very differently.
It just happened. I guess it was the luck of the draw. But it happens.
Accessibility Challenges
Participants described accessibility as the biggest limiting factor in their life. They talked about how physical and virtual environments are not designed for people with vision loss. While some accessibility measures have made navigating easier, many barriers remain. For example, most Internet content is not readable or accessible for people with vision loss, despite the introduction of screen readers and accessible websites.
I have my little reading machine. That's how I read—it's much easier than the reading machines they had years ago, which were so big, and you couldn't transport them easily.
Participants noted that mobility, transport, and access to information were the biggest frustrations. As a result, they described episodes of isolation, which could become more serious with changes in the physical environment, such as weather. Navigating in winter was mentioned as being particularly difficult. Participants also discussed the high costs associated with trying to modify their environment or obtain supports, describing examples of information technology and a tandem bike.
Impact on Family
Participants spoke about the commitment and sacrifice of loved ones who helped them adapt, plan, and organize. They talked about being dependent on their loved ones, which at times they found burdensome.
However, they also talked about how loved ones can develop a sense of responsibility and independence in being given tasks to accomplish that developed skills, such as money management and organization.
My daughter had to mature very quickly from when she was little, because she had to help Mom with all these things. So she learned how to shop, how to save, how to spend, and how to pay bills out of necessity, but it benefited her when she grew up and moved out of the house. Now she has rent and bills, and she understands that stuff. In exchange for that, she also had a lot more freedom, because she helped me with things.
Participants described everyday technology as both an enabler and a barrier: an enabler when it could assist with simple tasks (such as screen readers and apps), but a barrier when it was designed without visual impairment in mind (for example, kitchen appliances without buttons).
Process to Receive Retinal Prosthetics
Patients report being well-informed and clear about expectations and the procedure for receiving the Argus II system. Some patients mentioned doing a lot of research online before the procedure.
I wasn't really surprised, because before I had the surgery, I looked at a lot of information on the Internet. There's lots of information, and it seems there are a lot of people in other parts of the world who have already had this.
I knew that it certainly wasn't going to be restoring my vision to 20/20 again. And I knew that it was a different type of vision. I knew that it wouldn't be the same type of sight I had.
Participants described the implantation procedure as straightforward day surgery lasting 4 hours. Surgery was followed by a pain-free three-week recovery period. Once the glasses had been introduced, participants noticed immediate positive results.
As soon as I put my glasses on, I was able to see the lights on in the boardroom, the doctor, and my friends. I was like, “Oh my God.”
I was quite amazed that I could see all of the shapes sitting around the table, which was something that for many years I haven't been able to see.
Several learning sessions were provided to help teach objects and shapes. Learning these objects and shapes took at least several sessions. One patient mentioned the expectation that vision would keep improving as the brain adjusted to the prosthetic.
Impact of Argus II System on Quality of Life
Participants described Argus II as having a substantial impact on enabling the perception of light/dark and shapes/objects. While it was not the same as restoring full sight, it gave them the fundamental elements of sight, which was tremendously important in helping them navigate the physical environment and assisting with day-to-day activities such as mobility and eating. As a result, participants noted increased confidence. They also said they felt safer in their environment, indoors and outdoors.
It helps me in places like the subway, so I know where the doors are, when they open, and whether there is an empty seat instead of sitting in someone's lap. It also helps me when I eat at home or at a restaurant. Then I know I am able to find a fork or glass in the dining room.
I do find that I'm able to distinguish the difference between the grass and the pavement. I'm still not as good at it as I think I should be, but I'm getting better.
I'm pleased that I've got it, and I'm only looking forward to it getting better.
One patient also mentioned the increased social comfort that came from having the Argus II system.
I do find that, particularly in social situations, it's benefiting me, because at least I can see where people are. I can tell, for example, if I'm talking to somebody and they walk away, I know that they're gone, which I didn't before.”
Participants perceived the functionality provided by the Argus II system to be a great improvement from living a life without vision. They noted that while improvements were desirable—such as providing colour or greater detail—even the perception of light was impactful, helping them tell the difference between day and night and orient themselves.
Discussion
A number of important themes emerged from the interviews.
First, we confirmed that retinitis pigmentosa has a substantial effect on day-to-day functioning, especially when it comes to interacting with physical and virtual environments. This effect increased as the disease progressed. Despite societal efforts to enhance accessibility, physical and virtual environments can be functionally challenging for people with retinitis pigmentosa. Loss of opportunity was also described as a barrier, preventing people with retinitis pigmentosa from making choices that were possible for others (for example, job opportunities and other career-related choices). Still, participants were generally high-functioning, primarily because they had developed a “can-do” attitude and received substantial support from family, friends, and adaptive technologies. Participants had also invested heavily in assistive devices to help them navigate these environments.
Participants saw adaptation as a critical element of the day-to-day experience for people with retinitis pigmentosa. While there have been societal efforts to enable accessibility for people with low vision, participants saw greater success when they adapted by further orienting their living space to their own needs (for example, putting markers, buttons, and Braille in certain locations; keeping items in the same location; or purchasing items that could overcome obstacles in the physical environment, such as tandem bikes). People also adapted the virtual environment, using technologies such as screen readers or audio devices. However, adaptation came at great financial cost and was often only partially effective.
Finally, participants said the Argus II system provided important improvements in quality of life for people with retinitis pigmentosa. Participants reported being able to navigate their physical and social environment more easily and with more confidence. The procedure, recovery, and rehabilitation were all described as only minimally problematic.
Conclusions
Retinitis pigmentosa has a substantial effect on a person's quality of life, limiting opportunities and presenting accessibility challenges. The Argus II system can enable perception of light/dark and shapes/objects, providing people with the fundamental elements of vision. Using these informational gains, people with retinitis pigmentosa can orient themselves more easily in their environment, supporting the notion shared by one of the participants that “nobody should have to live in darkness forever.”
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
Based on evidence of moderate quality, the Argus II retinal prosthesis system improved visual function, real-life functional outcomes, and quality of life in patients with advanced retinitis pigmentosa. The Argus II system is costly, but the budget impact of publicly funding it would be small, because of the small number of eligible patients. The Argus II system can only enable perception of light/dark and shapes/objects, but these advancements represent important gains for people with retinitis pigmentosa in terms of mobility and quality of life.
Acknowledgments
The medical editor was Jeanne McKane; others involved in the development and production of this report were Tanveer Singh, Kellee Kaulback, Ana Lang, Claude Soulodre, Sarah McDowell, Andrée Mitchell, Vivian Ng, Anil Thota, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We are very grateful to the patients who shared their experiences with us. We are also grateful to Robert Devenyi for his expert opinion on retinitis pigmentosa and the Argus II retinal prosthesis system, and for data on the number of implants performed at the University Health Network, Toronto, Ontario.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted expert.
ABBREVIATIONS
- FLORA
Functional Low-vision Observer Rated Assessment
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- GVA
Grating visual acuity
- ICER
Incremental cost-effectiveness ratio
- NGVA
Non-grating visual acuity
- NICE
National Institute for Health and Care Excellence
- QALY
Quality-adjusted life-year
- VisQoL
Vision and Quality of Life Index
GLOSSARY
- Functional vision
The degree of vision necessary to perform basic life tasks.
- Incremental cost-effectiveness ratio (ICER)
Determines “a unit of benefit” for an intervention by dividing the incremental cost by the effectiveness. The incremental cost is the difference between the cost of the treatment under study and an alternative treatment. The effectiveness is usually measured as additional years of life or as “quality-adjusted life years.”
- LogMAR
A method of testing vision by standardizing the measurement of patients' ability to identify objects. The optometrist's eye chart is a common example of the LogMAR method.
- Observational study
A study that does not involve any intervention on the part of the investigator. Generally, investigators observe real world changes in health status in relation to changes in other patient characteristics.
- Prosthesis
A device designed to replace a missing body part. May be functional, cosmetic, or both.
- Quality-adjusted life-year (QALY)
A measurement that takes into account both the number of years gained by a patient from a procedure and the quality of those extra years (ability to function, freedom from pain, etc.). The QALY is commonly used as an outcome measure in cost–utility analyses.
- Retina
The part of the eye that receives information from the person's surroundings and converts it into electrical impulses that the brain interprets as sight.
- Retinal implant
A device that mimics the activity of the retina by receiving visual information and converting it to electrical impulses that create visual sensations in the brain. There are two types of implant. The epiretinal implant, which sits on the outer surface of the retina, and the subretinal implant, which is placed behind the retina. Each works by stimulating the nerve cells.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: February 9, 2017
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <January 2017>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to February 08, 2017>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2015>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2015>, Embase <1980 to 2017 Week 06>, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
exp Retinal Diseases/ (319821)
-
2
(retina* adj2 (disease* or degeneration)).ti,ab,kf. (24659)
-
3
exp Retinitis Pigmentosa/(16486)
-
4
((rod adj cone* adj (dystroph* or degenerat*)) or retinopath* pigment* or (tapetoretinal adj degeneration*) or ((retinitis or retinopath*) adj (pigmentosa* or pigmentary))).ti,ab,kf. (15276)
-
5
exp Vision Disorders/ (271745)
-
6
(micropsia* or visual impairment* or metamorphopsia* or visual disorder* or blindness or hemeralopia* or macropsia* or vision disorder* or vision disabilit* or amauros?s).ti,ab,kf. (76424)
-
7
or/1–6 (579577)
-
8
Visual Prosthesis/ (2144)
-
9
(((visual or retinal or epiretinal) adj (prosthes#s or implant*)) or bionic eye* or epiretinal device*).ti,ab,kf. (1923)
-
10
or/8–9 (3384)
-
11
7 and 10 (1356)
-
12
(Argus II or (Second Sight and (visual prosthes#s or Argus or medical product*)) or (argus adj6 (retin* or degenerat*))).ti,ab,kf. (141)
-
13
or/11–12 (1382)
-
14
exp Animals/ not Humans/ (15731102)
-
15
13 not 14 (806)
-
16
limit 15 to english language [Limit not valid in CDSR,DARE; records were retained] (683)
-
17
limit 16 to yr=“2015 -Current” [Limit not valid in DARE; records were retained] (119)
-
18
17 use ppez,cctr,coch,dare,clhta,cleed (114)
-
19
exp retina disease/ (203621)
-
20
(retina* adj2 (disease* or degeneration)).tw,kw. (25533)
-
21
exp retinitis pigmentosa/ (16486)
-
22
((rod adj cone* adj (dystroph* or degenerat*)) or retinopath* pigment* or (tapetoretinal adj degeneration*) or ((retinitis or retinopath*) adj (pigmentosa* or pigmentary))).tw,kw. (15379)
-
23
exp visual impairment/ (154997)
-
24
(micropsia* or visual impairment* or metamorphopsia* or visual disorder* or blindness or hemeralopia* or macropsia* or vision disorder* or vision disabilit* or amauros?s).tw,kw. (78680)
-
25
or/19–24 (389908)
-
26
exp Visual Prosthesis/ (2384)
-
27
(((visual or retinal or epiretinal) adj (prosthes#s or implant*)) or bionic eye* or epiretinal device*).tw,kw,dv. (1998)
-
28
or/26–27 (3482)
-
29
25 and 28 (1303)
-
30
(Argus II or (Second Sight and (visual prosthes#s or Argus or medical product*)) or (argus adj6 (retin* or degenerat*))).tw,kw,dv. (168)
-
31
or/29–30 (1335)
-
32
(exp animal/ or nonhuman/) not exp human/ (10056562)
-
33
31 not 32 (1127)
-
34
limit 33 to english language [Limit not valid in CDSR,DARE; records were retained] (973)
-
35
limit 34 to yr=“2015 -Current” [Limit not valid in DARE; records were retained] (253)
-
36
35 use emez (142)
-
37
18 or 36 (256)
-
38
37 use ppez (105)
-
39
37 use emez (142)
-
40
37 use coch (0)
-
41
37 use cctr (4)
-
42
37 use clhta (5)
-
43
37 use cleed (0)
-
44
37 use dare (0)
-
45
remove duplicates from 37 (186)
Grey Literature
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, ClinicalTrials.gov, Tufts/CEA Registry
Keywords used: Argus, second sight, visual prosthetic, visual prosthesis, visual implant, retinal prosthetic, retinal prosthesis, retinal implant, visual prosthe*, prothese visuel, implant visuel, retina
Results: 15
Economic Evidence Search
Economic Literature Search – Retinal Prosthetics for Retinitis Pigmentosa (Argus II) Update
Search requested by: Hong Anh Tu
Librarian: Corinne Holubowich
Search date: February 9, 2017
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <January 2017>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to February 08, 2017>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2015>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2015>, Embase <1980 to 2017 Week 06>, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
exp Retinal Diseases/ (319821)
-
2
(retina* adj2 (disease* or degeneration)).ti,ab,kf. (24659)
-
3
exp Retinitis Pigmentosa/ (16486)
-
4
((rod adj cone* adj (dystroph* or degenerat*)) or retinopath* pigment* or (tapetoretinal adj degeneration*) or ((retinitis or retinopath*) adj (pigmentosa* or pigmentary))).ti,ab,kf. (15276)
-
5
exp Vision Disorders/ (271745)
-
6
(micropsia* or visual impairment* or metamorphopsia* or visual disorder* or blindness or hemeralopia* or macropsia* or vision disorder* or vision disabilit* or amauros?s).ti,ab,kf. (76424)
-
7
or/1–6 (579577)
-
8
Visual Prosthesis/ (2144)
-
9
(((visual or retinal or epiretinal) adj (prosthes#s or implant*)) or bionic eye* or epiretinal device*).ti,ab,kf. (1923)
-
10
or/8–9 (3384)
-
11
7 and 10 (1356)
-
12
(Argus II or (Second Sight and (visual prosthes#s or Argus or medical product*)) or (argus adj6 (retin* or degenerat*))).ti,ab,kf. (141)
-
13
or/11–12 (1382)
-
14
economics/ (252825)
-
15
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (785385)
-
16
economics.fs. (389731)
-
17
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (733650)
-
18
exp “costs and cost analysis”/ (535914)
-
19
cost*.ti. (246772)
-
20
cost effective*.tw. (267904)
-
21
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (168484)
-
22
models, economic/ (167960)
-
23
markov chains/ or monte carlo method/ (67247)
-
24
(decision adj1 (tree* or analy* or model*)).tw. (36227)
-
25
(markov or markow or monte carlo).tw. (107891)
-
26
quality-adjusted life years/ (32930)
-
27
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (56703)
-
28
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (106988)
-
29
or/14–28 (2383490)
-
30
13 and 29 (45)
-
31
30 use ppez,cctr,coch,dare,clhta (11)
-
32
13 use cleed (1)
-
33
or/31–32 (12)
-
34
limit 33 to english language [Limit not valid in CDSR,DARE; records were retained] (12)
-
35
limit 34 to yr=“2015 -Current” [Limit not valid in DARE; records were retained] (4)
-
36
exp retina disease/ (203621)
-
37
(retina* adj2 (disease* or degeneration)).tw,kw. (25533)
-
38
exp retinitis pigmentosa/ (16486)
-
39
((rod adj cone* adj (dystroph* or degenerat*)) or retinopath* pigment* or (tapetoretinal adj degeneration*) or ((retinitis or retinopath*) adj (pigmentosa* or pigmentary))).tw,kw. (15379)
-
40
exp visual impairment/ (154997)
-
41
(micropsia* or visual impairment* or metamorphopsia* or visual disorder* or blindness or hemeralopia* or macropsia* or vision disorder* or vision disabilit* or amauros?s).tw,kw. (78680)
-
42
or/36–41 (389908)
-
43
exp Visual Prosthesis/ (2384)
-
44
(((visual or retinal or epiretinal) adj (prosthes#s or implant*)) or bionic eye* or epiretinal device*).tw,kw,dv. (1998)
-
45
or/43–44 (3482)
-
46
42 and 45 (1303)
-
47
(Argus II or (Second Sight and (visual prosthes#s or Argus or medical product*)) or (argus adj6 (retin* or degenerat*))).tw,kw,dv. (168)
-
48
or/46–47 (1335)
-
49
Economics/ (252825)
-
50
Health Economics/ or exp Pharmacoeconomics/ (223273)
-
51
Economic Aspect/ or exp Economic Evaluation/ (429321)
-
52
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (733650)
-
53
exp “Cost”/ (535914)
-
54
cost*.ti. (246772)
-
55
cost effective*.tw. (267904)
-
56
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (168484)
-
57
Monte Carlo Method/ (55006)
-
58
(decision adj1 (tree* or analy* or model*)).tw. (36227)
-
59
(markov or markow or monte carlo).tw. (107891)
-
60
Quality-Adjusted Life Years/ (32930)
-
61
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (56703)
-
62
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (106988)
-
63
or/49–62 (1976494)
-
64
48 and 63 (36)
-
65
64 use emez (27)
-
66
limit 65 to english language [Limit not valid in CDSR,DARE; records were retained] (27)
-
67
limit 66 to yr=“2015 -Current” [Limit not valid in DARE; records were retained] (9)
-
68
35 or 67 (13)
-
69
68 use ppez (3)
-
70
68 use emez (9)
-
71
68 use coch (0)
-
72
68 use cctr (0)
-
73
68 use clhta (1)
-
74
68 use cleed (0)
-
75
68 use dare (0)
-
76
remove duplicates from 68 (11)
Appendix 2: Clinical Evidence Quality Assessment
Our first consideration was study design; we started with the assumption that randomized controlled trials are high quality, whereas observational studies are low quality. We then took into account five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias. Limitations in these areas resulted in downgrading the quality of evidence. Finally, we considered three main factors that may raise the quality of evidence: the large magnitude of effect, the dose-response gradient, and any residual confounding factors. For more detailed information, please refer to the latest series of GRADE articles.14
Table A1:
GRADE Evidence Profile for Comparison of the Argus II Retinal Prosthesis System On and Off
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Visual Function: Object Localization | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Visual Function: Direction of Motion | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Visual Function: Grating Visual Acuity | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Orientation and Mobility (Find the Door) | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Orientation and Mobility (Follow the Line) | |||||||
| 1 (observational)19 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Visual Orientation | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Visual Mobility | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Daily Life | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Interaction With Others | |||||||
| 1 (observational)8 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Sock Sorting | |||||||
| 1 (observational)21 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Sidewalk Tracking | |||||||
| 1 (observational)21 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Functional Outcomes: Walking Direction Discrimination | |||||||
| 1 (observational)21 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Quality of Life: Injury | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Quality of Life: Life | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Quality of Life: Assistance | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Nonec | ⊕⊕ Low |
| Quality of Life: Roles | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
| Quality of Life: Activity | |||||||
| 1 (observational)20 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | Underlying trajectory of retinitis pigmentosa (+1)b | ⊕⊕⊕ Moderate |
Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Observational studies started with a low GRADE level because of inherent limitations in study design (e.g., lack of randomization, lack of blinding, and loss to follow-up). We did not lower the GRADE level further unless there were more substantial study limitations.
The natural history of retinitis pigmentosa is a progressive deterioration of vision, eventually leading to blindness. The Argus II retinal prosthesis system is the only treatment option currently available to restore partial functional vision for these patients.
Not upgraded for the assistance domain because of large standard error and small sample size (i.e., imprecision).
Table A2:
Risk of Bias Among Observational Studies for Comparison of the Argus II Retinal Prosthesis System On and Offa
| Confounding | Selection bias | Measurement bias | Publication bias | Reporting bias | Model misspecification | Other bias | |
|---|---|---|---|---|---|---|---|
| da Cruz et al, 201619 | N | N | ?b | N | Yc | N | N |
| Dagnelie et al, 201621 | N | N | ?b | N | N | N | N |
| Duncan et al, 201620 | N | N | ?d | N | N | N | N |
| Geruschat et al, 20168 | N | N | ?b | N | N | N | N |
Abbreviations: Y, Yes; N, No; ?, Unsure.
Risk of bias assessed using the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS)15
No masking of operators or evaluators, but it was logistically impossible to mask.
Out of 30 patients in the original cohort of the Argus II International Study, safety data were available for 27 and efficacy data were available for 21 to 22.
Although the VisQoL questionnaire23 was vision-specific, it has been constructed and validated in relatively few patients with profound vision loss. There was no alternative instrument specific to the small retinitis pigmentosa population when the study was begun.
Appendix 3: Detailed Breakdown of Argus II System Costsa
Table A3:
Argus II System Costs
| Argus II System | |||
| 1 | Argus II implant | Epiretinal implant and external Argus II components (glasses and video-processing unit) | $179,850 |
| 2 | Surgical procedure | Operating room supplies, including standard vitrectomy surgical supplies, Argus II surgical supplies (Ekhardt tips, 3083 sleeves, camera drape), and operating room nurse staff time for 4-hour procedure | $2,488 |
| 3 | Epiretinal replacement parts | Annual replacement of Argus II epiretinal implant parts | $1,100 |
| 4 | Eye exams (preoperative) |
|
$159 |
| 5 | University Health Network Argus II surgical instrument replacement | Annual replacement of surgical instruments specifically needed to implant the Argus II epiretinal device on the patient's retina, such as retinal tack forceps, silicone tip forceps | $1,320 |
| Low-Vision Rehabilitation | |||
| 6 | Eye exams (postoperative) | Technician staff time (20 minutes per test) to perform the following postoperative assessments in an Argus II patient:
|
$995 |
| 7 | Argus II system activation and fitting | Technician staff time to activate, calibrate, and fit Argus II system in a patient over six postoperative sessions. Continued adjustments to Argus II system provided to patient during low-vision rehabilitation (30 hours per patient per year) | $2,527 |
| 8 | Argus II training kits | Low-vision rehabilitation training kits for use in clinic and to take home to support use of Argus II system | $2,135 |
| 9 | Low-vision rehabilitation specialist | Occupational therapist staff time to perform 10 low-vision rehabilitation sessions, some in the clinic and some in the patient's home or workplace, and/or public settings, based on patient preference (1 hour per session) | $1,618 |
| 10 | Patient coordinator | Patient coordinator staff time to coordinate patient scheduling of eye exam assessments, day surgery visit, and Argus II implant orders, as well as providing patient education and support; the coordinator also serves as a liaison between the manufacturer (Second Sight, Inc.), the patient, and the UHN clinical team (50 hours per patient per year) | $4,814 |
| 11 | University Health Network Argus II equipment maintenance | Annual maintenance of UHN Argus II equipment for clinician fitting system, psychophysical test system, and communication adapter system | $7,171 |
| Total cost to provide one Argus II system | $204,177 | ||
Abbreviation: University Health Network.
Cost data were provided in the submission for the review of Argus II (expert opinion).
Appendix 4: Letter of Information and Consent and Release Form
LETTER OF INFORMATION
Health Quality Ontario is conducting a review of the Argus II retinal prosthesis system for patients with retinitis pigmentosa. The purpose is to understand whether this therapy should be more broadly funded in Ontario.
An important part of this review involves speaking to patients and families of those who have experience with retinitis pigmentosa, who may or may not have received the Argus II retinal prosthesis sytem. Our goal is to make sure the experiences of patients and caregivers are considered in the funding recommendations for the Argus II treatment for retinitis pigmentosa.
WHAT DO YOU NEED FROM ME?
-
✓
20–40 minutes of your time for a phone or in-person interview to share your story
-
✓
Permission to audio- (not video-) record the interview
WHAT YOUR PARTICIPATION INVOLVES
If you agree to share your experiences, you will be asked to have an interview with Health Quality Ontario staff. The interview will likely last 20–40 minutes. It will be held in a private location or over the telephone. With your con sent, the interview will be audio-taped. The interviewer will ask you questions about you or your loved one's condition and your perspectives about retinitis pigmentosa treatment options in Ontario. If you have received the Argus II retinal prosthesis system, the interviewer will ask some additional questions surrounding the procedure.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before your interview. Withdrawal will in no way affect care you receive.
CONFIDENTIALITY
All information collected for the review will be kept confidential and privacy will be protected except as required by law. The results of this review will be published, however no identifying information will be released or published. Any records containing information from your interview will be stored securely.
RISKS TO PARTICIPATION:
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their lived experience. If this is the case, please contact any staff.
If you are interested in participating, please contact Health Quality Ontario staff:
Consent and Release Form
This form is to be read and completed in accordance with the following instructions before it can be signed.
-
1.
I,_____ allow Health Quality Ontario (Ontario Health Quality Council) to use to inform the development of an evidence based review:
Check off all appropriate boxes:
-
a)
___a recording of my voice
-
b)
___a quotation or summary of my opinion that I expressed during an interview
-
e)
___name & contact information
-
a)
-
2.
Please read the following paragraphs before affixing your signature under section 3.
-
a)
Personal information collected pursuant to, and on this form, will be used for purposes described on this form and for no other purpose. Health Quality Ontario (Ontario Health Quality Council) acknowledges that you have provided this personal information freely and voluntarily. If you have any questions about this collection of this personal information, contact:
-
b)
By signing this form as indicated below, you agree to hereby release and forever discharge the Health Quality Ontario (Ontario Health Quality Council), its officers, employees, agents and representatives from any and all claims, demands, expenses, actions, causes of action and for any and all liability howsoever caused, arising out of, or in any way related to the collection, use and disclosure of information, recordings and images authorized to be collected pursuant to, or on this form.
-
c)
By signing this form as indicated below, you agree to forever waive any and all rights that you may have to the use of information and recordings that are authorized to be collected pursuant to, or on this form; and you acknowledge that all information, recordings and images shall hereafter remain the exclusive property of the Health Quality Ontario (Ontario Health Quality Council).
-
a)
-
3.
Signature is to be affixed in the appropriate space provided below.
I have read this form after it was completed, I understand and agree to be bound by its contents, and I am eighteen (18) years of age or over.
Signature_____
Print name_____
Date_____
Appendix 5: Interview Guide
interview for Argus II HTA
Intro
Explain HQO purpose, HTA process, and purpose of interview
History of Retinitis Pigmentosa - diagnosis and background (general only)
Lived- Experience
Day-to-day routine
What is the impact on quality of life? Effect on loved-ones/caregivers?
Interventions
Experiences with interventions prior to Angus II? How well could you manage your condition with available therapies? Other treatment/therapies used and associated costs/barriers?
Argus II
Expectations of Argus? Decision-making to choose this intervention?
Role of family in decision-making? Physician? Other sources of information (Internet)?
Contrast emotion (anxiety, worry) vs logic? As this applies to risk and side-effects?
Procedure
Post-op rehabilitation and how long after the implant could you start to regain vision?
Quality of life after? New activities?
After implant, do you need any further treatment? Any maintenance costs? Drawback or limitations?
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The clinical epidemiologist was Christine Lee, the health economist was Hong Anh Tu, the patient, caregiver, and public engagement specialist was David Wells, and the medical librarian was Corinne Holubowich.
KEY MESSAGES
What Is This Health Technology Assessment About?
Retinitis pigmentosa is an eye disease people are born with. People who have it slowly lose their vision. The Argus II retinal implant is the only treatment approved by Health Canada for retinitis pigmentosa. A device is implanted in a patient's eye, and it works with a special set of glasses to restore some vision to people with this disease.
In 2016, Health Quality Ontario published a health technology assessment of the Argus II system. In the current report, we updated our assessment based on evidence published since then. We assessed the clinical benefits and harms of the Argus II system, value for money, and the budget impact of publicly funding it. We also interviewed people who have used it to learn more about their experiences.
What Did This Health Technology Assessment Find?
The Argus II system helps people see better and is generally safe to use. It is costly, but because retinitis pigmentosa is rare, the budget impact of publicly funding it would be $0.71 to $0.78 million per year over the next 5 years, assuming 4 implants per year. Vision loss creates many barriers that have a substantial effect on people's quality of life. The Argus II system can return some elements of vision to people with advanced retinitis pigmentosa, an important gain for them in terms of independence and accessibility.
Contributor Information
Health Quality Ontario:
Christine Lee, Hong Anh Tu, David Wells, and Corinne Holubowich
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Health Quality Ontario. Retinal prosthesis system for advanced retinitis pigmentosa: a health technology assessment. Ont Health Technol Assess Ser. 2016; 16 (14): 1–63. [PMC free article] [PubMed] [Google Scholar]
- (2).Health Quality Ontario. Retinal prosthesis system for advanced retinitis pigmentosa: OHTAC recommendation [Internet]. Toronto (ON): Queen's Printer for Ontario; 2016. Available from: http://www.hqontario.ca/Portals/0/Documents/evidence/reports/recommendation-retinal-prosthetics-en-1606.pdf [Google Scholar]
- (3).Grover S, Fishman GA, Anderson RJ, Tozatti MS, Heckenlively JR, Weleber RG, et al. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. 1999; 106 (9): 1780–5. [DOI] [PubMed] [Google Scholar]
- (4).Hayeems RZ, Geller G, Finkelstein D, Faden RR. How patients experience progressive loss of visual function: a model of adjustment using qualitative methods. Br J Ophthalmol. 2005; 89 (5): 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006; 1: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Retinitis pigmentosa [Internet]. Toronto (ON): Foundation Fighting Blindness; c2015. [cited 2017 Mar 3]. Available from: http://ffb.ca/wp-content/uploads/2015/12/Retinitis-pigmentosa.pdf [Google Scholar]
- (7).Luo YH, Fukushige E, Da Cruz L. The potential of the second sight system bionic eye implant for partial sight restoration. Expert Rev Med Devices. 2016; 13 (7): 673–81. [DOI] [PubMed] [Google Scholar]
- (8).Geruschat DR, Richards TP, Arditi A, da Cruz L, Dagnelie G, Dorn JD, et al. An analysis of observer-rated functional vision in patients implanted with the Argus II retinal prosthesis system at three years. Clin Exp Optom. 2016; 99 (3): 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Centre for Medicare and Medicaid Services. January 2017. update of the ambulatory surgical center (ASC) payment system [Internet]. Baltimore (MD): U.S. Department of Health and Human Services; 2016. [cited 2017 Mar 3]. Available from: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9923.pdf [Google Scholar]
- (10).CPT category III codes code descriptors [Internet]. Chicago (IL): American Medical Association; c2016. [cited 2017 March 3]. Available from: https://www.ama-assn.org/sites/default/files/media-browser/public/cpt/cptcat3-desc-january2017.pdf [Google Scholar]
- (11).NHS England to fund bionic eye surgery [Internet]. Wakefield (UK): U.K. National Health Service; 2016. [cited 2017 March 3]. Available from: https://www.england.nhs.uk/2016/12/bionic-eye-surgery/ [Google Scholar]
- (12).Second Sight announces positive reimbursement renewal decision in Germany [Internet]. Wayne (PA): Bryn Mawr Communications, LLC.; c2017. [cited 2017 March 3]. Available from: http://eyewiretoday.com/2017/02/02/second-sight-announces-positive-reimbursement-renewal-decision-in-germany [Google Scholar]
- (13).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016; 75: 40–6. [DOI] [PubMed] [Google Scholar]
- (14).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64 (4): 380–2. [DOI] [PubMed] [Google Scholar]
- (15).Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66 (4): 408–14. [DOI] [PubMed] [Google Scholar]
- (16).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151 (4): 264–9. [DOI] [PubMed] [Google Scholar]
- (17).Insertion of an epiretinal prosthesis for retinitis pigmentosa [Internet]. London (UK): National Institute for Health and Care Excellence; 2015. [cited 2017 Mar 3]. Available from: https://www.nice.org.uk/guidance/ipg519 [Google Scholar]
- (18).Agency for Healthcare Research and Quality. Retinal prostheses in the Medicare population [Internet]. Rockvile (MD): U.S. Department of Health and Human Services; 2016. [cited 2017 Mar 3]. Available from: https://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id103TA.pdf [Google Scholar]
- (19).da Cruz L, Dorn JD, Humayun MS, Dagnelie G, Handa J, Barale PO, et al. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology. 2016; 123 (10): 2248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Duncan JL, Richards TP, Arditi A, da Cruz L, Dagnelie G, Dorn JD, et al. Improvements in vision-related quality of life in blind patients implanted with the Argus II epiretinal prosthesis. Clin Exp Optom. 2016; 100 (2): 144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dagnelie G, Christopher P, Arditi A, da Cruz L, Duncan JL, Ho AC, et al. Performance of real-world functional vision tasks by blind subjects improves after implantation with the Argus(R) II retinal prosthesis system. Clin Exp Ophthalmol. 2016; 45 (2): 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Geruschat DR, Flax M, Tanna N, Bianchi M, Fisher A, Goldschmidt M, et al. FLORA: Phase I development of a functional vision assessment for prosthetic vision users. Clin Exp Optom. 2015; 98 (4): 342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Peacock S, Misajon R, Iezzi A, Richardson J, Hawthorne G, Keeffe J. Vision and quality of life: development of methods for the VisQoL vision-related utility instrument. Ophthalmic Epidemiol. 2008; 15 (4): 218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Schover L, Odensky E, Martinetti P. Developing an internet-based intervention for cancer-related male sexual problems. Psychooncology. 2015; 24: 2–3. [Google Scholar]
- (25).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013; 16 (2): 231–50. [DOI] [PubMed] [Google Scholar]
- (26).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada, 4th ed [Internet]. Ottawa (ON): The Agency; 2017. [cited 2017 Apr]. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [Google Scholar]
- (27).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada, 3rd ed [Internet]. Ottawa (ON): The Agency; 2006. [cited 2015 Sep]. Available from: https://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf [Google Scholar]
- (28).Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel JA, Stanga PE, et al. Interim results from the international trial of Second Sight's visual prosthesis. Ophthalmology. 2012; 119 (4): 779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford (UK): Oxford University Press; 2006. [Google Scholar]
- (30).Na KH, Kim HJ, Kim KH, Han S, Kim P, Hann HJ, et al. Prevalence, age at diagnosis, mortality, and cause of death in retinitis pigmentosa in Korea: a nationwide population-based study. Am J Ophthalmol. 2017; 176: 157–65. [DOI] [PubMed] [Google Scholar]
- (31).Statistics Canada. Life tables, Canada, provinces and territories 2009 to 2011 [Internet]. Ottawa (ON): Statistics Canada; 2017. [updated 2017; cited 2017 Feb 20]. Available from: http://www.statcan.gc.ca/pub/84-537-x/84-537-x2013005-eng.htm [Google Scholar]
- (32).Brown MM, Brown GC, Sharma S, Kistler J, Brown H. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001; 85 (3): 327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc. 1999; 97: 473–511. [DOI] [PubMed] [Google Scholar]
- (34).Frick KD, Roebuck MC, Feldstein JI, McCarty CA, Grover LL. Health services utilization and cost of retinitis pigmentosa. Arch Ophthalmol. 2012; 130 (5): 629–34. [DOI] [PubMed] [Google Scholar]
- (35).Bank of Canada [Internet]. Ottawa (ON): Bank of Canada; 2015. [updated 2015; cited 2015 Aug 22]. Available from: http://www.bankofcanada.ca/ [Google Scholar]
- (36).Statistics Canada. Consumer price index, health and personal care by province (Canada) [Internet]. Ottawa (ON): Statistics Canada; 2016. [updated 2016 Jan 22; cited 2016 Mar 22]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/I01/econ161a-eng.htm [Google Scholar]
- (37).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. The patient. 2011; 4 (1): 1–10. [DOI] [PubMed] [Google Scholar]
- (38).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. The patient. 2012; 5 (3): 199–211. [DOI] [PubMed] [Google Scholar]
- (39).OHTAC Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Toronto (ON): Queen's Printer for Ontario; 2015. [Google Scholar]
- (40).Tjornhoj-Thomsen T, Hansen HP. Knowledge in health technology assessment: who, what, how? Int J Technol Assess Health Care. 2011; 27 (4): 324–9. [DOI] [PubMed] [Google Scholar]
- (41).Rowe G, Frewer LJ. A typology of public engagement mechanisms. Sci Technol Hum Val. 2005; 30 (2): 251–90. [Google Scholar]
- (42).Brundisini F, Giacomini M, DeJean D, Vanstone M, Winsor S, Smith A. Chronic disease patients' experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2013; 13 (15): 1–33. [PMC free article] [PubMed] [Google Scholar]
- (43).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (44).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage Publications; 1999. p. 33–45. [Google Scholar]
- (45).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods Thousand Oaks (CA): Sage Publications; 1994. p. 23–41. [Google Scholar]
- (46).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage Publications; 2002. [Google Scholar]
- (47).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage Publications; 1998. [Google Scholar]
- (48).Health Technology Assessment international Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. 2015. [updated 2015]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf
- (49).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990; 13 (1): 3–21. [Google Scholar]
- (50).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]