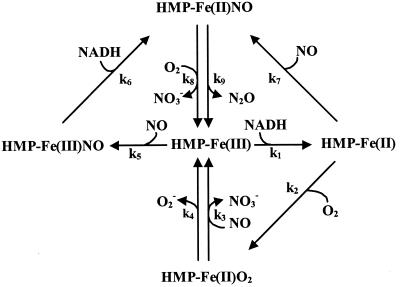
Figure 4.
Competitive substrate binding in the catalytic cycle of HMP. Ferric HMP can react with either NADH (k1) or NO (k5) to form ferrous HMP or ferric-nitrosyl HMP, respectively. The latter is reduced by NADH to ferrous-nitrosyl HMP (k6). NO (k7) and O2 (k2) compete for ferrous HMP to form the ferrous-nitrosyl or oxy forms, respectively. NO wins out under biologically relevant conditions (see text). The oxy and ferrous-nitrosyl forms complete the cycle by reacting with NO (k3) or O2 (k8), respectively, to generate nitrate and ferric HMP. Because of HMP's higher affinity for NO than O2, and because NO binds both ferric and ferrous heme whereas O2 only binds the ferrous form, the reaction will proceed mainly through the nitrosyl (denitrosylase) pathway (k5 or k1/k7). The slow oxidase (k4) and anaerobic NO reductase (k9) reactions are also depicted, although only the latter is known to have functional significance (9, 11). Reverse reactions and internal electron transfer steps were omitted for clarity. The nitrosyl HMP spectra seen during turnover may be interpreted in terms of a resonance between HMP(FeII)NO ⇄ HMP(Feδ+III)NOδ−, and the position of the relative charge may be dependent on O2. Higher pO2 would increase the FeIII character of the heme through formation of a “dioxynitrosyl” intermediate.