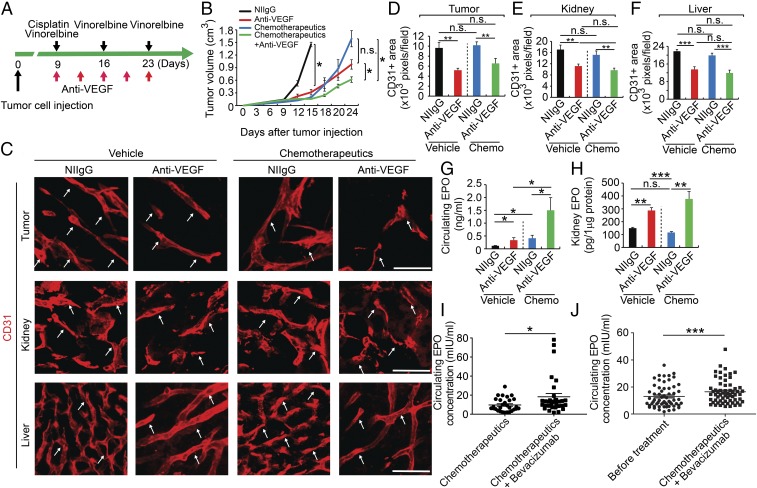
Fig. 6.
Chemotherapy in combination of anti-VEGF therapy elevated EPO expression. (A) Treatment schedule with anti-VEGF drug in combination of cisplatin and vinorelbine. (B) Tumor growth rates (n = 5 animals per group). (C) Immunohistochemical analyses of CD31+ microvessels Arrows point to CD31+ blood vessels. (Scale bars: 50 μm.) (D–F) Quantifications of CD31+ vessel density in tumor, kidney, and liver (n = 8 random fields per group). (G) ELISA analysis of plasma EPO protein levels (n = 5 samples per group). (H) ELISA analysis of kidney EPO protein levels (n = 4–5 samples per group). (I) ELISA analysis of plasma EPO protein levels of bevacizumab plus chemotherapeutics (9.31 ± 1.17 mIU) and chemotherapeutics alone-treated (18.32 ± 3.44 mIU) colorectal cancer patients (n = 34 patients in chemotherapy alone group; n = 32 patients in bevacizumab plus chemotherapy group). (J) ELISA analysis of plasma EPO protein levels of control (12.5 ± 0.107 mIU) and bevacizumab plus chemotherapeutic docetaxel-epirubicin–treated (16.8 ± 0.124 mIU) breast cancer patients (n = 61 patients per group). *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Data are means ± SEM.