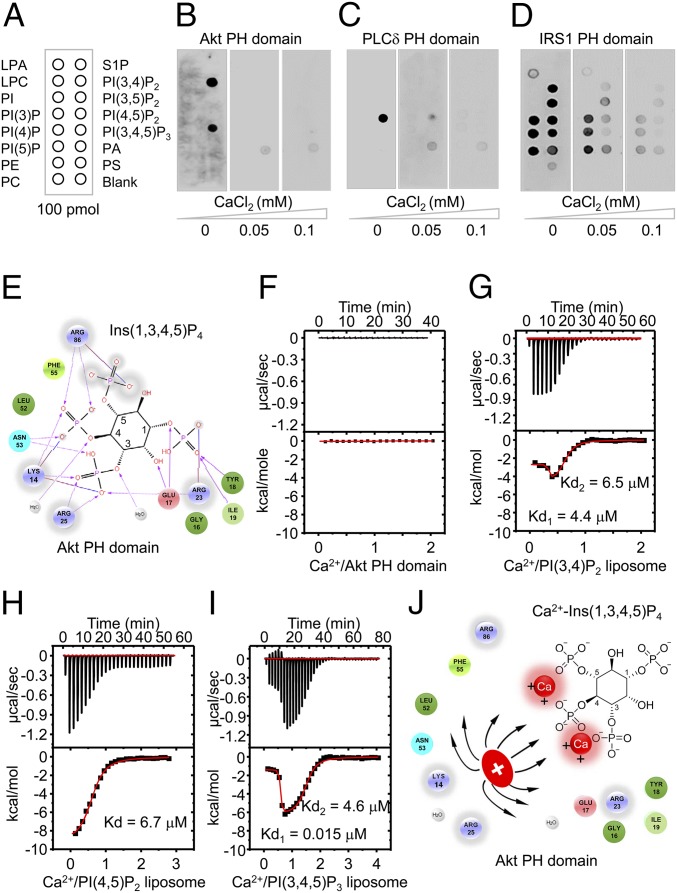
Fig. 4.
High Ca2+ concentrations abolishes the electrostatic interactions between PH domains and PIPs through the formation of Ca2+-PIPs. (A) Schematic representation of the various biological phospholipids (PIP strips, Echelon Biosciences), including LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; and S1P, sphingosine 1-phosphate. (B–D) Binding of the PH domains of Akt (B), PLCδ (C), and IRS1 (D) to immobilized phospholipids under the indicated Ca2+ concentrations. (E) Schematic representation of electrostatic interactions between the PH domain of Akt (Protein Data Bank accession code:1H10) and Ins(1,3,4,5)P4. (F–I) ITC results for Ca2+ binding to the PH domain of Akt (F), PI(3,4)P2 (G), PI(4,5)P2 (H), or PI(3,4,5)P3 (I) liposomes. Kd values were determined by curve fitting. (J) Schematic representation of electrostatic repulsion between basic residues in the Akt PH domain and Ca2+-Ins(1,3,4,5)P4. Positive charges of basic residues in the PH domain will repel the positively charged Ca2+-Ins(1,3,4,5)P4 and thus inhibit the electrostatic interactions in E.