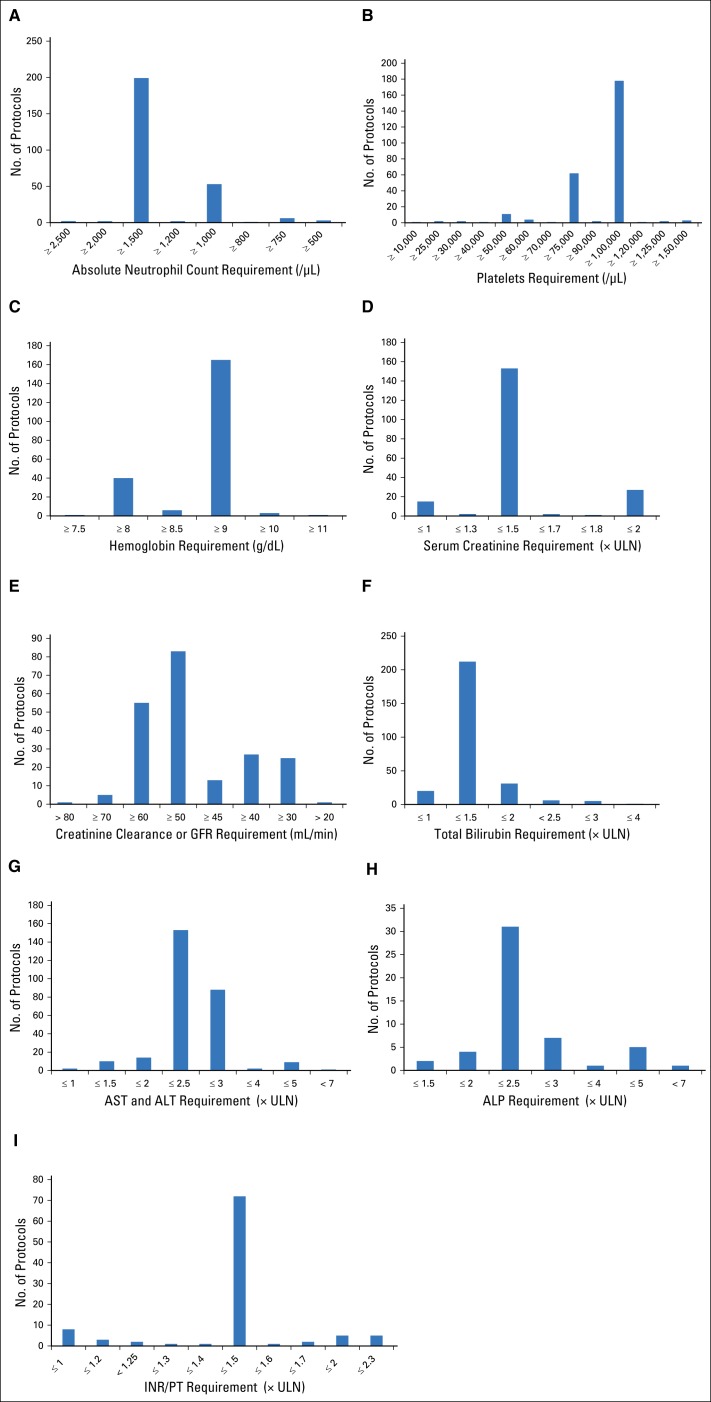
Fig 1.
Frequency of laboratory value requirements for 297 oncology investigational new drug protocols. Protocol-specified accepted laboratory test values and number of protocols with each requirement for (A) absolute neutrophil count (ANC), (B) platelet count, (C) hemoglobin, (D) serum creatinine, (E) creatinine clearance or glomerular filtration rate (GFR), (F) total bilirubin, (G) AST and ALT, (H) alkaline phosphatase (ALP), and (I) international normalized ratio (INR) or prothrombin time (PT). ULN, upper limit of normal.