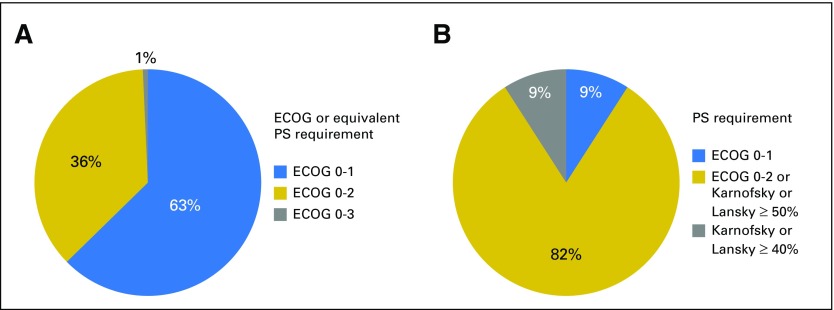
Fig 2.
Performance status (PS) requirements for oncology investigational new drug (IND) protocols. (A) Proportion of PS requirements for 284 adult protocols with Eastern Cooperative Oncology Group (ECOG) or equivalent (Karnofsky or other) criteria. (B) Proportion of PS requirements for 11 pediatric protocols.